Boi-Ogi-To, a Traditional Japanese Kampo Medicine, Promotes Cellular Excretion of Chloride and Water by Activating Volume-Sensitive Outwardly Rectifying Anion Channels
- PMID: 40341625
- PMCID: PMC12059615
- DOI: 10.1096/fj.202403278R
Boi-Ogi-To, a Traditional Japanese Kampo Medicine, Promotes Cellular Excretion of Chloride and Water by Activating Volume-Sensitive Outwardly Rectifying Anion Channels
Abstract
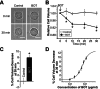
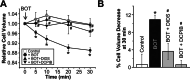
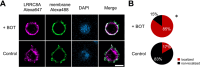
The Japanese Kampo medicine Boi-ogi-to (BOT) is known as an effective therapeutic agent for edema and nephrosis by promoting the excretion of excess body fluids. Despite its empirical effectiveness, scientific evidence supporting its effectiveness remains limited. In this study, we conducted a retrospective study of the effects of BOT administration on the blood test values of patients before and after taking the drug to attempt translational research between basic science and daily clinical practice by focusing on the molecular mechanism of action of BOT in vitro. We found that blood sodium and chloride levels are higher after taking BOT by analyzing the clinical test values before and after taking the drug from 28 patients attending Akita University Hospital. In this light, we measured the cell volume of human embryonic kidney HEK293T cells in vitro in order to investigate the possibility that BOT induces Cl- excretion and cell volume reduction. BOT showed concentration-dependent cell volume reduction with an EC50 of 686 μg/mL. The volume reduction effect was suppressed by the Cl- channel inhibitors DIDS and DCPIB. Furthermore, patch-clamp studies showed that BOT-activated Cl- currents exhibit outward rectification and time-dependent inactivation upon depolarization. These biophysical properties of BOT-induced Cl- currents correspond to those of volume-sensitive outward rectifier (VSOR) anion channels. The Cl- currents activated by the administration of BOT were inhibited by applying DIDS, DCPIB, and siRNA targeting the gene of LRRC8A, a core component of the VSOR channel, as well as in LRRC8-deficient cells. Additionally, BOT-induced Cl- currents were restored by coexpression of LRRC8A/C in LRRC8-deficient cells. Also, BOT was found to translocate LRRC8A proteins to the plasma membrane. These results demonstrated that BOT activates LRRC8-containing VSOR channels by delivering LRRC8A to the plasma membrane and induces Cl- release, thereby promoting water excretion.
Keywords: Boi‐Ogi‐to (BOT); VSOR; cell volume regulation; edema; herbal medicine; translational science.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Declaration of Transparency and Scientific Rigor: This declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigor of preclinical research recommended by funding agencies, publishers, and other organizations engaged with supporting research.
The authors declare no conflicts of interest.
Figures





Similar articles
-
Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel.Pflugers Arch. 2016 May;468(5):795-803. doi: 10.1007/s00424-015-1786-1. Epub 2016 Jan 8. Pflugers Arch. 2016. PMID: 26743872
-
Specific and essential but not sufficient roles of LRRC8A in the activity of volume-sensitive outwardly rectifying anion channel (VSOR).Channels (Austin). 2017 Mar 4;11(2):109-120. doi: 10.1080/19336950.2016.1247133. Epub 2016 Oct 20. Channels (Austin). 2017. PMID: 27764579 Free PMC article.
-
Distinct contributions of LRRC8A and its paralogs to the VSOR anion channel from those of the ASOR anion channel.Channels (Austin). 2017 Mar 4;11(2):167-172. doi: 10.1080/19336950.2016.1230574. Epub 2016 Aug 31. Channels (Austin). 2017. PMID: 27579940 Free PMC article.
-
Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor.Am J Physiol. 1997 Sep;273(3 Pt 1):C755-89. doi: 10.1152/ajpcell.1997.273.3.C755. Am J Physiol. 1997. PMID: 9316396 Review.
-
Molecular Identities and ATP Release Activities of Two Types of Volume-Regulatory Anion Channels, VSOR and Maxi-Cl.Curr Top Membr. 2018;81:125-176. doi: 10.1016/bs.ctm.2018.07.004. Epub 2018 Aug 17. Curr Top Membr. 2018. PMID: 30243431 Review.
References
-
- Lee J.‐Y., Yoon S.‐Y., Won J., Kim H.‐B., Kang Y., and Oh S. B., “Sinomenine Produces Peripheral Analgesic Effects via Inhibition of Voltage‐Gated Sodium Currents,” Neuroscience 358 (2017): 28–36. - PubMed
MeSH terms
Substances
Grants and funding
- 21K06778/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K06792/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H05842/MEXT | Japan Society for the Promotion of Science (JSPS)
- 2024/Akita University, Re-challenge Promotion Expenses
- 2023/Japan Kampo Medicine Education Foundation grant number
LinkOut - more resources
Full Text Sources

