TRPV2 mediates stress resilience in mouse cardiomyocytes
- PMID: 40341813
- PMCID: PMC12062233
- DOI: 10.1038/s42003-025-08167-9
TRPV2 mediates stress resilience in mouse cardiomyocytes
Abstract
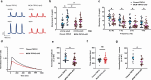
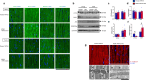
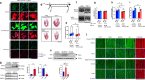
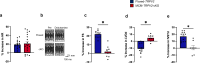
The heart dynamically compensates for haemodynamic stress, but how this resilience forms during cardiac growth is not clear. Using a temporally inducible, cardiac-specific knockout in mice we show that the Transient receptor potential vanilloid family 2 (TRPV2) channel is crucial for the maturation of cardiomyocyte stress resilience. TRPV2 defects in growing hearts lead to small morphology, abnormal intercalated discs, weak contractility, and low expression of serum response factor and Insulin-like growth factor-1 (IGF-1) signalling. Individual cardiomyocytes of TRPV2-deficient hearts show reduced contractility with abnormal Ca2+ handling. In cultured neonatal cardiomyocytes, mechanical Ca2+ response, excitation-contraction coupling, sarcoplasmic reticulum Ca2+ content, actin formation, nuclear localisation of Myocyte enhancer factor 2c, and IGF-1 expression require TRPV2. TRPV2-deficient hearts show a defective response to dobutamine stress and no compensatory hypertrophic response to phenylephrine administration, but no stress response to pressure overload. These data suggest TRPV2 mediates the maturation of cardiomyocyte stress resilience, and will advance therapeutic interventions and drug discovery for heart disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

