Role of polar localization of the silicon transporter OsLsi1 in metalloid uptake by rice roots
- PMID: 40341966
- PMCID: PMC12089981
- DOI: 10.1093/plphys/kiaf196
Role of polar localization of the silicon transporter OsLsi1 in metalloid uptake by rice roots
Abstract
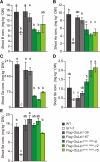
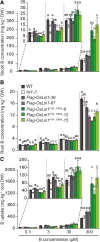
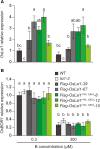
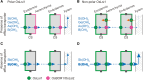
Low silicon (Si) rice 1 (OsLsi1) is a key transporter mediating Si uptake in rice (Oryza sativa). It is polarly localized at the distal side of the root exodermis and endodermis. Although OsLsi1 is also permeable to other metalloids, such as boron (B), germanium (Ge), arsenic (As), antimony (Sb), and selenium (Se), the role of its polar localization in the uptake of these metalloids remains unclear. In this study, we investigated the role of OsLsi1 polar localization in metalloid uptake by examining transgenic rice plants expressing polarly or nonpolarly localized OsLsi1 variants. Loss of OsLsi1 polar localization resulted in decreased accumulation of Ge, B, and As in shoots but increased Sb accumulation, while Se accumulation remained unaffected under normal conditions. Experiments with varying B concentrations revealed that B uptake is significantly lower at low B concentrations (0.3 to 3 μm) but higher at high B concentrations (300 μm) in plants expressing nonpolarly localized OsLsi1, despite the similar B permeability of both OsLsi1 variants in Xenopus oocytes and their comparable protein abundance in roots. Additionally, the loss of OsLsi1 polarity did not affect the abundance, localization, or high B-induced degradation of the borate transporter 1 (OsBOR1), an efflux transporter that cooperates with OsLsi1 for B uptake. Taken together, our findings demonstrate that the polar localization of OsLsi1 plays a critical role in regulating metalloid uptake, depending on the presence or absence of efflux transporters cooperating with OsLsi1.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. The authors declare no conflict of interest.
Figures



Similar articles
-
Boron uptake in rice is regulated post-translationally via a clathrin-independent pathway.Plant Physiol. 2022 Mar 4;188(3):1649-1664. doi: 10.1093/plphys/kiab575. Plant Physiol. 2022. PMID: 34893892 Free PMC article.
-
Polar localization of a rice silicon transporter requires isoleucine at both C- and N-termini as well as positively charged residues.Plant Cell. 2023 May 29;35(6):2232-2250. doi: 10.1093/plcell/koad073. Plant Cell. 2023. PMID: 36891818 Free PMC article.
-
Cell-Type-Dependent but CME-Independent Polar Localization of Silicon Transporters in Rice.Plant Cell Physiol. 2022 May 16;63(5):699-712. doi: 10.1093/pcp/pcac032. Plant Cell Physiol. 2022. PMID: 35277719
-
Transporters involved in mineral nutrient uptake in rice.J Exp Bot. 2016 Jun;67(12):3645-53. doi: 10.1093/jxb/erw060. Epub 2016 Feb 29. J Exp Bot. 2016. PMID: 26931170 Review.
-
Functions and transport of silicon in plants.Cell Mol Life Sci. 2008 Oct;65(19):3049-57. doi: 10.1007/s00018-008-7580-x. Cell Mol Life Sci. 2008. PMID: 18560761 Free PMC article. Review.
Cited by
-
On the move, direction matters: Polar localization of OsLsi1 for differential uptake of metalloids in rice.Plant Physiol. 2025 Jul 3;198(3):kiaf262. doi: 10.1093/plphys/kiaf262. Plant Physiol. 2025. PMID: 40578307 Free PMC article. No abstract available.
References
-
- Eaton FM. Deficiency, toxicity and accumulation of boron in plants. J Agric Res. 1944:69:237–279.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

