Targeting vascular endothelial growth receptor-2 (VEGFR-2): structural biology, functional insights, and therapeutic resistance
- PMID: 40341988
- PMCID: PMC12106596
- DOI: 10.1007/s12272-025-01545-1
Targeting vascular endothelial growth receptor-2 (VEGFR-2): structural biology, functional insights, and therapeutic resistance
Abstract
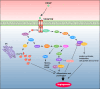
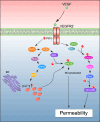
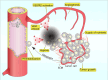
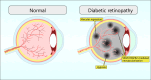
Angiogenesis, the process of new blood vessel formation, is a fundamental physiological process implicated in several pathological disorders. The vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are crucial for angiogenesis and vasculogenesis. Among them, the tyrosine kinase receptor VEGFR-2 is primarily expressed in endothelial cells (ECs). These cells regulate various physiological responses, including differentiation, cell proliferation, migration, and survival, by binding to VEGF mitogens. Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) is a key regulator of this process, making it a prime target for therapeutic intervention. Several drugs targeting VEGFR-2 have been approved and are currently utilized to halt the pathological axis of VEGF-VEGFR. This review will focus on the recent developments in the molecular structure and function of VEGFR-2, the molecular mechanism of VEGFR-2 activation, and its downstream signaling pathway. It will also discuss therapies and experimental drugs approved to inhibit the function of VEGFR-2 and the resistance mechanism.
Keywords: Angiogenesis; Pathology; Resistance; Signaling; VEGF; VEGFR-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing financial interests.
Figures



References
-
- Abdullah SE, Perez-Soler R (2012) Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer 118:3455–3467. 10.1002/cncr.26540 - PubMed
-
- Ahmad A, Nawaz MI (2022) Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem 123:1938–1965. 10.1002/jcb.30344 - PubMed
-
- Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438:946–953. 10.1038/nature04480 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

