Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
- PMID: 40342021
- PMCID: PMC12081034
- DOI: 10.3892/ijmm.2025.5545
Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
Abstract
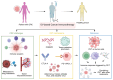
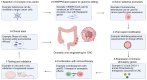
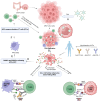
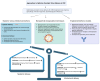
Colorectal cancer (CRC) is a leading health issue and treatments to eradicate it, such as conventional chemotherapy, are non‑selective and come with a number of complications. The present review focuses on the relatively new area of precision oncolytic viral therapy (OVT), with genetic targeting and immune modifications that offer a new future for CRC treatment. In the present review, an overview of the selection factors that are considered optimal for an oncolytic virus, mechanisms of oncolysis and immunomodulation applied to the OVT, as well as new strategies to improve the efficacy of this method are described. Additionally, cause‑and‑effect relationships are examined for OVT efficacy, mediated by the tumor microenvironment, and directions for genetic manipulation of viral specificity are explored. The possibility of synergy between OVT and immune checkpoint inhibitors and other treatment approaches are demonstrated. Incorporating the details of the present review, biomarker‑guided combination therapies in precision OVT for individualized CRC care, significant issues and future trends in this required area of medicine are highlighted. Increasingly, OVT is leaving the experimental stage and may become routine practice; it provides a new perspective on overcoming CRC and highlights the importance of further research and clinical work.
Keywords: biomarkers; colorectal cancer; immune modulation; oncolytic viral therapy; personalized treatment; precision medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
