Fluorescent Deferoxamine Complexes of Cu(II) and Zr(IV): Insights in the Development of Dual Imaging Probes
- PMID: 40342180
- PMCID: PMC12188159
- DOI: 10.1002/chem.202501203
Fluorescent Deferoxamine Complexes of Cu(II) and Zr(IV): Insights in the Development of Dual Imaging Probes
Abstract
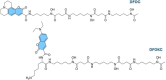
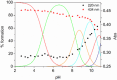
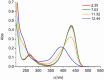
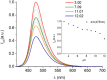
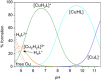
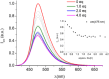
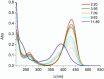
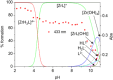
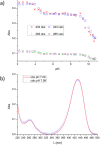
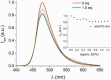
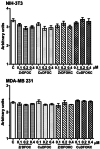
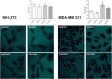
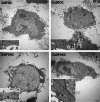
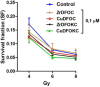
Deferoxamine (DFO) is widely regarded as benchmark chelator for 89Zr(IV), a commonly used PET (positron emission tomography) tracer. We have introduced a novel fluorescent coumarin derivative of DFO (DFOKC), characterized by chelating unit and fluorophore covalently linked via a lysine molecule. This design introduces a free primary amine group, which, in perspective, can be functionalized with biological vectors, potentially improving tumor tissue selectivity. Its acid-base and metal coordination properties toward Cu(II) and Zr(IV) ions were thoroughly characterized using UV-Vis and fluorescence emission spectroscopy. DFOKC strongly coordinates both metal ions, forming somewhat more stable complexes than DFO, while retaining fluorescence emission, thus enabling dual-mode optical and PET imaging. Biodistribution assays conducted on NIH-3T3 fibroblasts, and MDA-MB-231 mammary adenocarcinoma cell lines demonstrated that the presence of primary amine groups favors Zr-DFOKC complex cell internalization via pinocytosis compared to the parent molecule DFOC, in which the fluorophore is linked to the amine group of DFO. Furthermore, crystal violet and MTT assays revealed no cytotoxic effects or mitochondrial impairment, even at concentrations higher than those typically used for radio-diagnostic applications. These results strongly support the potential of DFOKC as a versatile and promising tool for dual imaging, offering significant advantages in molecular imaging.
Keywords: Cu(II) complexes; Zr(IV) complexes; deferoxamine; fluorescent PET probes; optical/PET dual mode imaging.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Li Z., Conti P. S., Adv. Drug Delivery Rev. 2010, 62, 1031. - PubMed
-
- O'Connor J. P., Aboagye E. O., Adams J. E., Aerts H. J., Barrington S. F., Beer A. J., Boellaard R., Bohndiek S. E., Brady M., Brown G., Buckley D. L., Chenevert T. L., Clarke L. P., Collette S., Cook G. J., deSouza N. M., Dickson J. C., Dive C., Evelhoch J. L., Faivre‐Finn C., Gallagher F. A., Gilbert F. J., Gillies R. J., Goh V., Griffiths J. R., Groves A. M., Halligan S., Harris A. L., Hawkes D. J., Hoekstra O. S., et al., Nat. Rev. Clin. Oncol. 2017, 14, 169. - PMC - PubMed
-
- Tarkin J. M., Joshi F. R., Rudd J. H., Nat. Rev. Cardiol. 2014, 11, 443. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

