Neurogrit Gold Attenuates 6-OHDA-Induced Dopaminergic Neurodegeneration in Parkinson's Model of Caenorhabditis elegans by Reducing α-Synuclein Accumulation and Pink/Pdr-1 Driven Mitochondrial Dysfunction
- PMID: 40342222
- PMCID: PMC12059624
- DOI: 10.1111/cns.70401
Neurogrit Gold Attenuates 6-OHDA-Induced Dopaminergic Neurodegeneration in Parkinson's Model of Caenorhabditis elegans by Reducing α-Synuclein Accumulation and Pink/Pdr-1 Driven Mitochondrial Dysfunction
Abstract
Introduction: Parkinson's disease (PD) is a neurodegenerative disorder majorly associated with movement and behavioral disturbances. Pathologically, the loss of dopaminergic (DA) neurons triggered by the deposition of α-synuclein (SNCA) leads to the decrease in dopamine levels affecting motor and cognitive functions of the brain. Current pharmacotherapy for PD only addresses its symptoms but is not able to halt its progression. Traditional medicines are being increasingly used for the treatment of neurodegenerative disorders.
Aim: The present study investigated the effects of Neurogrit Gold (NG), a herbo-mineral prescription medicine, on a Parkinson's model of Caenorhabditis elegans.
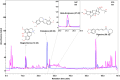
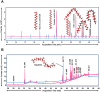
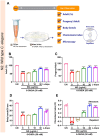
Methods: Chemical characterization of NG was performed on HPLC and GC-MS/MS platforms. Evaluation of NG was done in the neurotoxicant 6-OHDA-induced N2, BZ555, and NL5901 strains of C. elegans.
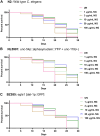
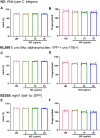
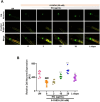
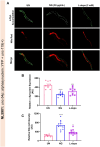
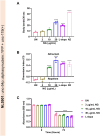
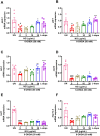
Results: It was observed that NG treatment did not hamper the lifespan, survival, and progeny development of C. elegans strains. The worms treated with NG were able to resist the deleterious effects of 6-OHDA on survival, progeny development, body bends, and chemotaxis in N2 and DA neuron degeneration in BZ555 worms. In NL5901 worms, NG treatment reduced SNCA aggregation, restored lipid content, as well as improved body bends, chemotaxis, and food uptake. Gene expression studies on 6-OHDA exposed and NG-treated N2 worms suggest that the neuroprotective effects of NG stem from its ability to regulate genes involved in mitochondrial autophagy (pink-1, pdr-1); dopamine synthesis (cat-2); redox (sod-3) and protein folding homeostasis (hsf-1, hsp-12.3).
Conclusion: Neurogrit Gold has robust neuroprotective effects, making it a suitable treatment option against etiologies of Parkinson's disease.
Keywords: Caenorhabditis elegans; 6‐OHDA; Neurogrit gold; Parkinson's disease; autophagy; levodopa; mitochondria.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The test article (NG) was sourced from Divya Pharmacy, Haridwar, India. Acharya Balkrishna is an honorary trustee in Divya Yog Mandir Trust, which governs Divya Pharmacy, Haridwar. In addition, he holds an honorary managerial position in Patanjali Ayurved Ltd., Haridwar, India. Divya Pharmacy, Haridwar, India, and Patanjali Ayurved Ltd., Haridwar, India, manufacture and sell herbal medicinal products. Other than providing the test formulation (NG), Divya Pharmacy was not involved in any aspect of the research reported in this study. All other authors have declared no conflicts of interest.
Figures
References
-
- Elbaz A., Carcaillon L., Kab S., and Moisan F., “Epidemiology of Parkinson's Disease,” Revue Neurologique (Paris) 172, no. 1 (2016): 14–26. - PubMed
-
- Mishra A. K., Ur Rasheed M. S., Shukla S., Tripathi M. K., Dixit A., and Singh M. P., “Aberrant Autophagy and Parkinsonism: Does Correction Rescue From Disease Progression?,” Molecular Neurobiology 51, no. 3 (2015): 893–908. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

