Clinical Features and Outcomes of Glutamic Acid Decarboxylase-65 Antibody-Associated Pure Cerebellar Ataxia and Stiff Person Syndrome Spectrum Disorders: A Single-Center Cohort Study
- PMID: 40342250
- PMCID: PMC12059465
- DOI: 10.1111/ene.70166
Clinical Features and Outcomes of Glutamic Acid Decarboxylase-65 Antibody-Associated Pure Cerebellar Ataxia and Stiff Person Syndrome Spectrum Disorders: A Single-Center Cohort Study
Abstract
Background: Cerebellar ataxia is associated with greater disability in glutamic acid decarboxylase-65 (GAD65) antibody-associated neurological disorders and can occur in isolation or as part of stiff person syndrome (SPS) spectrum disorders (SPSD). We sought to phenotypically characterize this subpopulation with cerebellar dysfunction.
Methods: Observational study of GAD65 antibody-seropositive individuals with cerebellar involvement seen at Johns Hopkins (1997-2024). Subjects were divided into two groups based on cerebellar dysfunction in the presence (SPSD; SPS-plus and progressive encephalomyelitis with rigidity and myoclonus [PERM]) or absence (pure cerebellar ataxia [pCA]) of classic SPS features. Clinical and paraclinical findings were analyzed descriptively.
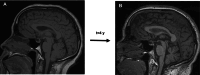
Results: Seventy-two patients were selected among 356 (62 SPSD, 10 pCA). Mean age for patients with pCA was 58 ± 16 years versus 46 ± 15 years for SPSD (p = 0.012). Males comprised 50% of the pCA group versus 19% SPSD (p = 0.049). High GAD65 antibody serum titers occurred in 76% without group differences, while cerebrospinal fluid antibody positivity occurred in 35/37 (95%) of SPSD versus 5/8 (62%) pCA (p = 0.033). Although the modified Rankin scale was similar (median 3, interquartile range 2-4) in both groups, the brief ataxia rating scale indicated a higher burden of cerebellar abnormalities in pCA versus SPSD, and there was a trend toward greater cerebellar atrophy by MRI in pCA (p = 0.44). Rituximab and benzodiazepine use was more frequent in SPSD versus pCA.
Conclusions: GAD65 antibody-associated ataxia is disabling irrespective of accompanying SPS features. Patients with pCA were older, more commonly male, and may have more frequent cerebellar atrophy than those with SPSD. Prospective validation of cerebellar outcomes and neuroimaging findings is needed.
Keywords: GAD‐65; autoimmune neurology; cerebellar ataxia; neuroimmunology; stiff person syndrome.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
J.D.K., A.M., H.R.C., H.A., A.E., and D.D.L. declare no conflicts of interest. Y.W. has received consultant fees from TG Therapeutics and has received research funding (paid directly to institution) from Genentech and uniQure. S.D.N. has received consultant fees for scientific advisory boards from Biogen, Genentech, Bristol Myers Squibb, Novartis, and TG Therapeutics, is the study lead PI for a Roche clinical trial program, and has received research funding (paid directly to institution) from Biogen, Roche, Lundbeck, Genentech, Sanofi, The Stiff Person Syndrome Research Foundation, National Multiple Sclerosis Society, Department of Defense, and Patient Centered Outcomes Research Institute.
Figures




References
-
- Moersch F. P. and Woltman H. W., “Progressive Fluctuating Muscular Rigidity and Spasm (“Stiff‐Man” Syndrome); Report of a Case and Some Observations in 13 Other Cases,” Proceedings of the Staff Meetings. Mayo Clinic 38, no. 15 (1956): 421–427. - PubMed
-
- Barker R. A., Revesz T., Thom M., Marsden C. D., and Brown P., “Review of 23 Patients Affected by the Stiff Man Syndrome: Clinical Subdivision Into Stiff Trunk (Man) Syndrome, Stiff Limb Syndrome, and Progressive Encephalomyelitis With Rigidity,” Journal of Neurology, Neurosurgery, and Psychiatry 65, no. 5 (1998): 633–640, 10.1136/jnnp.65.5.633. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

