HIV-Tat upregulates the expression of senescence biomarkers in CD4+ T-cells
- PMID: 40342418
- PMCID: PMC12058733
- DOI: 10.3389/fimmu.2025.1568762
HIV-Tat upregulates the expression of senescence biomarkers in CD4+ T-cells
Abstract
Introduction: Current antiretroviral therapy (ART) for HIV infection reduces plasma viral loads to undetectable levels and has increased the life expectancy of people with HIV (PWH). However, this increased lifespan is accompanied by signs of accelerated aging and a higher prevalence of age-related comorbidities. Tat (Trans-Activator of Transcription) is a key protein for viral replication and pathogenesis. Tat is encoded by 2 exons, with the full-length Tat ranging from 86 to 101 aa (Tat101). Introducing a stop codon in position 73 generates a 1 exon, synthetic 72aa Tat (Tat72). Intracellular, full-length Tat activates the NF-κB pro-inflammatory pathway and increases antiapoptotic signals and ROS generation. These effects may initiate a cellular senescence program, characterized by cell cycle arrest, altered cell metabolism, and increased senescence-associated secretory phenotype (SASP) mediator release However, the precise role of HIV-Tat in inducing a cellular senescence program in CD4+ T-cells is currently unknown.
Methods: Jurkat Tetoff cell lines stably transfected with Tat72, Tat101, or an empty vector were used. Flow cytometry and RT-qPCR were used to address senescence biomarkers, and 105 mediators were assessed in cell supernatants with an antibody-based membrane array. Key results obtained in Jurkat-Tat cells were addressed in primary, resting CD4+ T-cells by transient electroporation of HIV-Tat-FLAG plasmid DNA.
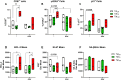
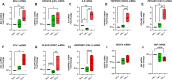
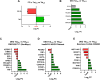
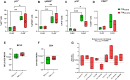
Results: In the Jurkat cell model, expression of Tat101 increased the levels of the senescence biomarkers BCL-2, CD87, and p21, and increased the release of sCD30, PDGF-AA, and sCD31, among other factors. Tat101 upregulated CD30 and CD31 co-expression in the Jurkat cell surface, distinguishing these cells from Tat72 and Tetoff Jurkats. The percentage of p21+, p16+, and γ-H2AX+ cells were higher in Tat-expressing CD4+ T-cells, detected as a FLAG+ population compared to their FLAG- (Tat negative) counterparts. Increased levels of sCD31 and sCD26 were also detected in electroporated CD4+ T-cell supernatants.
Discussion: Intracellular, full-length HIV-Tat expression increases several senescence biomarkers in Jurkat and CD4+ T-cells, and SASP/Aging mediators in cell supernatants. Intracellular HIV-Tat may initiate a cellular senescence program, contributing to the premature aging phenotype observed in PWH.
Keywords: CD4+ T-Cell; HIV; HIV-Tat; SASP; aging; cellular senescence.
Copyright © 2025 Casanova, Rodríguez-Agustín, Ayala-Suárez, Moraga, Maleno, Mallolas, Martínez, Sánchez-Palomino, Miró, Alcamí and Climent.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

