European survey on CAR T-Cell analytical methods from apheresis to post-infusion immunomonitoring
- PMID: 40342422
- PMCID: PMC12058815
- DOI: 10.3389/fimmu.2025.1567582
European survey on CAR T-Cell analytical methods from apheresis to post-infusion immunomonitoring
Abstract
Background: Chimeric Antigen Receptor (CAR) T-cell therapy has emerged as a revolutionary approach to cancer treatment. Given the rapid expansion of new indications addressed by newly developed CAR T-cell products, it is essential to standardize analytical methods for the characterization/monitoring of apheresis materials, drug products, and post-infusion patient samples.
Methods: The T2Evolve Consortium, part of the European Union's Innovative Medicines Initiative (IMI), conducted an extensive anonymous online survey between February and June 2022. Comprising 36 questions, the survey targeted a wide range of stakeholders involved in engineered T-cell therapies, including researchers, manufacturers, and clinicians. Its goal was to address the current variability within the CAR T-cell field, focusing on analytical assays for quality control of apheresis materials, drug products, and post-infusion immunomonitoring. Another objective was to identify gaps and needs in the field.
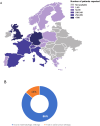
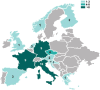
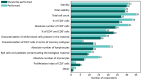
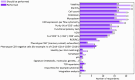
Results: A total of 53 respondents from 13 european countries completed the survey, providing insights into the most commonly used assays for apheresis material and drug product characterization, alongside safety and efficacy tests required by the Pharmacopeia. Notably, a minority of respondents conducted phenotypical characterization of T-cell subsets in the drug product and assessed activation/exhaustion T cell profiles.
Conclusion: The survey underscored the necessity to standardize CAR T-cell functional potency assays and identify predictive biomarkers for response, relapse, and toxicity. Additionally, responses indicated significant variability in CAR T-cell monitoring during short-term patient follow-up across clinical centers. This European survey represents the first initiative to report current approaches in different stages of CAR T-cell therapies via a survey, from drug product quality controls to post-infusion immunomonitoring. Based on these findings, and with input from T2EVOLVE experts, the next step will be to address harmonization in the identified areas. These efforts are anticipated to significantly enhance cancer patients' access to engineered T cell therapy safely and effectively throughout Europe.
Keywords: CAR T-cells; European survey; T2Evolve; immunomonitoring; leukapheresis; lymphodepleting chemotherapy.
Copyright © 2025 De Angelis, D’Amore, Lecot, Neininger, Lorrain, Gambotti, Dreuillet, Courcault, Chatterjee, Delgado, Galy, Franz, Rodriguez-Madoz, Cabrerizo, Richter, Girvalaki, Noviello, Tassi, Sanges, Luu, Hudecek, Kremer, Locatelli, Negre and Quintarelli.
Conflict of interest statement
MH and MLu are inventors on patents related to CAR T-cell therapy filed by the University of Würzburg. BD, CQ and FL are inventors on patents related to CAR T-cell therapy filed by Bambino Gesù Children’s Hospital. Authors KN, MLo were employed by Information Technology for Translational Medicine S.A. Authors SC was employed by 4Takeda Development Center Americas, Inc and Takeda Pharmaceuticals U.S.A., Inc., Authors AR was employed by Miltenyi Biotec GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. . CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. (2021) 27:1797–805. doi: 10.1038/s41591-021-01497-1 - DOI - PMC - PubMed
-
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia; chimeric antigen receptor-modified T cells for acute lymphoid leukemia; chimeric antigen receptor T cells for sustained remissions in leukemia. New England J Med. 368(16):1509–18. doi: 10.1056/NEJMx160005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

