High-mobility group protein B1 derived mutant peptide mB Box-97 inhibits the formation of neutrophil extracellular traps
- PMID: 40342425
- PMCID: PMC12059481
- DOI: 10.3389/fimmu.2025.1565252
High-mobility group protein B1 derived mutant peptide mB Box-97 inhibits the formation of neutrophil extracellular traps
Abstract
Introduction: Neutrophil Extracellular Traps (NETs) are vital for innate immunity, playing a key role in controlling pathogen and biofilm proliferation. However, excessive NETosis is implicated in autoimmunity, inflammatory and neoplastic diseases, as well as thrombosis, stroke, and post-COVID-19 complications. Managing NETosis, therefore is a significant area of ongoing research. Herein, we have identified a peptide derived from HMGB1 that we have modified via a point mutation that is referred to as mB Box-97. In our recent study in a murine lung infection model, mB Box-97 was shown to be safe and effective at disrupting biofilms without eliciting an inflammatory response typically associated with HMGB1. Here we show that the lack of an inflammatory response of mB Box-97 is in part due to the inhibition of NETosis of which we investigated the mechanism of action.
Methods: mB Box-97's anti-NETosis activity was assessed using human neutrophils with known NET inducers PMA, LPS, or Ionomycin. Additionally, mB Box-97's binding to Protein Kinase C (PKC), in addition to downstream effects on NADPH oxidase (NOX) activation, Reactive Oxygen Species (ROS) generation and thereby NETosis were assessed.
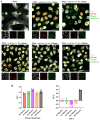
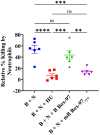
Results: mB Box-97 significantly inhibited NETosis regardless of the type of induction pathway. Mechanistically, mB Box-97 inhibits PKC activity likely through direct binding and thereby reduced downstream activities including NOX activation, ROS production and NETosis.
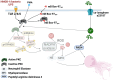
Conclusions: mB Box-97 is a promising dual acting therapeutic candidate for managing NET-mediated pathologies and resolving biofilm infections. Our results reveal that PKC is a viable target for NETosis inhibition independent of NET inducer and worthy of further study. These findings pave the way for a novel class of therapeutics aimed at controlling excessive NETosis, potentially offering new treatments for a range of inflammatory and immune-related diseases.
Keywords: COVID-19; HMGB1; NET inhibition; autoimmunity; inflammation; therapeutic.
Copyright © 2025 More, Devaraj, Robledo-Avila, Partida-Sanchez, Bakaletz and Goodman.
Conflict of interest statement
LB and SG have equity stakes in Clarametyx Biosciences, Inc., of which they are founders, shareholders, and members of the scientific advisory board. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

