Case Report: Two cases of chemotherapy refractory aggressive variant prostate cancer with extreme durable response to PARP inhibitor
- PMID: 40342821
- PMCID: PMC12058769
- DOI: 10.3389/fonc.2025.1533627
Case Report: Two cases of chemotherapy refractory aggressive variant prostate cancer with extreme durable response to PARP inhibitor
Abstract
Background: Aggressive variant prostate cancer (AVPC) represents a distinct clinical subset characterized by resistance to novel hormone therapies and an unfavorable prognosis, frequently associated with the concurrent loss of tumor suppressor genes (TSG) such as PTEN, RB1, and TP53. While the progression-free survival (PFS) and overall survival (OS) of AVPC are relatively short, the optimal first-line treatment remains unclear.
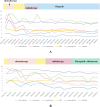
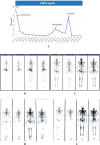
Presentation: In this case report, we presented two de novo AVPC cases who have ultimately benefited from the usage of PARP inhibitors. The first patient was a 64-year-old male who was diagnosed during prostate biopsy featured by mutations in PTEN, and loss of RB1, BRCA2, ATM, and FANCA. He was treated with docetaxel/albumin-bound paclitaxel and cisplatin in the first line. Second-line therapy was applied with radiotherapy and Olaparib after failure of first-line therapy, resulting in a PSA response sustained for three years. The second case was a 75-year-old male with localized neuroendocrine feature and mutations in TP53, loss of RB1 and HDAC2. He was treated with sustained ADT and chemotherapy in the first-line treatment. Radiotherapy and Fluzoparib + abiraterone was applied as subsequent treatments with a PSA response for 2 years.
Conclusions: These two cases demonstrating a satisfactorily durable response to PARP inhibitors indicating its clinical benefit in AVPC population with detected DNA damage response (DDR) defects. The survival improvement with PARP inhibitors observed in our clinical experiences, along with current advances in tumor sequencing provide more information on future clinical trials and explorations of innovative therapies in AVPC population.
Keywords: PARP inhibitor; aggressive variant prostate cancer; chemotherapy; prostate cancer; radiotherapy; treatment.
Copyright © 2025 Jiang, Bi, Chen, Bi, Deng and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting.Int J Mol Sci. 2025 May 31;26(11):5309. doi: 10.3390/ijms26115309. Int J Mol Sci. 2025. PMID: 40508118 Free PMC article.
-
Prognostic Expression Signature of RB1, PTEN, and TP53 Genes in Patients with Metastatic Hormone-sensitive Prostate Cancer Treated with Androgen Receptor Pathway Inhibitors.Eur Urol Open Sci. 2024 Oct 21;70:86-90. doi: 10.1016/j.euros.2024.10.008. eCollection 2024 Dec. Eur Urol Open Sci. 2024. PMID: 39502104 Free PMC article.
-
The Emerging Predictive and Prognostic Role of Aggressive-Variant-Associated Tumor Suppressor Genes Across Prostate Cancer Stages.Int J Mol Sci. 2025 Jan 1;26(1):318. doi: 10.3390/ijms26010318. Int J Mol Sci. 2025. PMID: 39796175 Free PMC article. Review.
-
Concordance and Clinical Significance of Genomic Alterations in Progressive Tumor Tissue and Matched Circulating Tumor DNA in Aggressive-variant Prostate Cancer.Cancer Res Commun. 2023 Nov 3;3(11):2221-2232. doi: 10.1158/2767-9764.CRC-23-0175. Cancer Res Commun. 2023. PMID: 37877742 Free PMC article.
-
[Aggressive variant prostate cancer and transdifferentiated neuroendocrine prostate cancer: from diagnosis to therapy].Urologie. 2025 Mar;64(3):246-255. doi: 10.1007/s00120-024-02511-3. Epub 2025 Feb 10. Urologie. 2025. PMID: 39928109 Review. German.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

