Understanding the role of oxylipins in Cannabis to enhance cannabinoid production
- PMID: 40343123
- PMCID: PMC12058684
- DOI: 10.3389/fpls.2025.1568548
Understanding the role of oxylipins in Cannabis to enhance cannabinoid production
Abstract
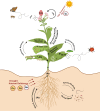
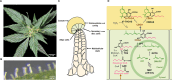
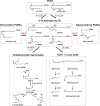
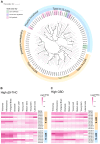
Phytocannabinoids are medically important specialized defense compounds that are sparsely distributed among plants, yet Cannabis sativa can synthesize unprecedented amounts of these compounds within highly specialized surface cell factories known as glandular trichomes. The control mechanisms that allow for this high level of productivity are poorly understood at the molecular level, although increasing evidence supports the role of oxylipin metabolism in phytocannabinoid production. Oxylipins are a large class of lipid-based oxygenated biological signaling molecules. Although some oxylipins are known to participate in plant defense, roles for the majority of the ca. 600 plant oxylipins are largely unknown. In this review, we examine oxylipin gene expression within glandular trichomes and identify key oxylipin genes that determine the fate of common lipid precursors. Mechanisms by which oxylipins may be interacting with phytocannabinoid metabolism, as well as specialized plant metabolism more broadly, are discussed and a model summarizing these contributions proposed.
Keywords: glandular trichomes; green leaf volatiles; jasmonates; lipoxygenase; oxylipins; specialized metabolites.
Copyright © 2025 Senevirathne, Gendall, Johnson and Welling.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Oxylipin biosynthetic gene families of Cannabis sativa.PLoS One. 2023 Apr 26;18(4):e0272893. doi: 10.1371/journal.pone.0272893. eCollection 2023. PLoS One. 2023. PMID: 37099560 Free PMC article.
-
Relative contribution of LOX10, green leaf volatiles and JA to wound-induced local and systemic oxylipin and hormone signature in Zea mays (maize).Phytochemistry. 2020 Jun;174:112334. doi: 10.1016/j.phytochem.2020.112334. Epub 2020 Mar 13. Phytochemistry. 2020. PMID: 32172019
-
Characterization of the Cannabis sativa glandular trichome epigenome.BMC Plant Biol. 2024 Nov 14;24(1):1075. doi: 10.1186/s12870-024-05787-x. BMC Plant Biol. 2024. PMID: 39538149 Free PMC article.
-
The Oxylipin Pathways: Biochemistry and Function.Annu Rev Plant Biol. 2018 Apr 29;69:363-386. doi: 10.1146/annurev-arplant-042817-040440. Epub 2017 Nov 20. Annu Rev Plant Biol. 2018. PMID: 29166128 Review.
-
Oxylipin metabolism in response to stress.Curr Opin Plant Biol. 2002 Jun;5(3):230-6. doi: 10.1016/s1369-5266(02)00250-9. Curr Opin Plant Biol. 2002. PMID: 11960741 Review.
References
-
- Anderson L. L., Ametovski A., Luo J. L., Everett-Morgan D., McGregor I. S., Banister S. D., et al. . (2021). Cannabichromene, related phytocannabinoids, and 5-fluoro-cannabichromene have anticonvulsant properties in a mouse model of Dravet syndrome. ACS Chem. Neurosci. 12, 330–339. doi: 10.1021/acschemneuro.0c00677 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

