Noninvasive prenatal testing: an overview
- PMID: 40343140
- PMCID: PMC12055489
- DOI: 10.18773/austprescr.2025.019
Noninvasive prenatal testing: an overview
Abstract
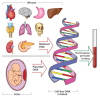
Australian health authorities recommend offering prenatal screening for fetal chromosome conditions, also known as aneuploidies (e.g. Down syndrome [trisomy 21]), to all pregnant individuals to support informed decision-making. Noninvasive prenatal testing (NIPT) is one of 3 types of prenatal aneuploidy screening tests available in Australia. NIPT requires a maternal blood test after 10 weeks gestation. Although it doesn't require an ultrasound, a 12- or 13-week ultrasound is recommended as it provides an opportunity for early diagnosis of major structural anomalies. NIPT is not subsidised by Medicare. It is important to take a patient-centred approach when discussing screening options. Patients should be encouraged to consider whether knowing the test result will impact their pregnancy decision-making or preparations. There are two main NIPT approaches: genome-wide and targeted. All currently available NIPT platforms perform well for detecting the common autosomal aneuploidies (trisomy 21, 18 and 13). NIPT has the highest true-positive rate (highest sensitivity) and lowest false-positive rate (highest specificity) among aneuploidy screening methods, however false-positive results can occur. Genetic counselling and confirmatory invasive diagnostic testing are recommended for patients with a high-probability NIPT result, especially if they are considering pregnancy termination.
Keywords: Down syndrome; aneuploidy screening; cell-free fetal DNA; noninvasive prenatal testing; prenatal screening.
(c) Therapeutic Guidelines.
Conflict of interest statement
Conflicts of interest: Alice Poulton is employed by Monash IVF Group, which owns a noninvasive prenatal testing (NIPT) brand (NEST plus). Alice receives funding from The University of Melbourne, the Murdoch Children's Research Institute and Monash IVF Group for her PhD on preimplantation genetic testing for monogenic conditions and attendance at conferences to present her research. This funding does not support the research of NEST plus performance or outcomes. Lisa Hui has received a clinical investigator grant from the Medical Research Future Fund (MRFF) for the project ‘Closing the critical knowledge gaps in perinatal genomics’. She has also received a grant from the Australian Research Council (ARC) for the project ‘Ethical, societal and regulatory aspects of advanced genomic testing’. These projects include research on the ethics, consumer and clinician perspectives of prenatal screening, including NIPT. MRFF and ARC are funded by the Australian Government. Lisa is Elected Board Director of the International Society for Prenatal Diagnosis (ISPD) and Associate Editor of Prenatal Diagnosis. Lisa is the first author of the ISPD 2023 position statement on the use of NIPT in singleton pregnancies, and Chair of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists’ guideline development group for ‘Screening and diagnosis of fetal structural anomalies and chromosome conditions’.
Figures
References
-
- Living Evidence for Australian Pregnancy and Postnatal care (LEAPP) Guidelines Group. Australian Pregnancy Care Guidelines. Australian Living Evidence Collaboration. https://livingevidence.org.au/living-guidelines/leapp/#pregnancy-guidelines [cited 2024 Sep 27]
-
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Screening and diagnosis of fetal structural anomalies and chromosome conditions. 2024. https://ranzcog.edu.au/resources/statements-and-guidelines-directory/ [cited 2025 Mar 5]
-
- Rose NC, Barrie ES, Malinowski J, Jenkins GP, McClain MR, LaGrave D, et al. ACMG Professional Practice and Guidelines Committee . Systematic evidence-based review: The application of noninvasive prenatal screening using cell-free DNA in general-risk pregnancies. Genet Med 2022;24:1992. 10.1016/j.gim.2022.07.002 - DOI - PubMed
-
- Lindquist A, Hui L, Poulton A, Kluckow E, Hutchinson B, Pertile MD, et al. State-wide utilization and performance of traditional and cell-free DNA-based prenatal testing pathways: the Victorian Perinatal Record Linkage (PeRL) study. Ultrasound Obstet Gynecol 2020;56:215-24. 10.1002/uog.21899 - DOI - PubMed
Further reading
-
- Hui L, Ellis K, Mayen D, Pertile MD, Reimers R, Sun L, et al. Position statement from the International Society for Prenatal Diagnosis on the use of non-invasive prenatal testing for the detection of fetal chromosomal conditions in singleton pregnancies. Prenat Diagn 2023;43:814-28. 10.1002/pd.6357 - DOI - PubMed
-
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Screening and diagnosis of fetal structural anomalies and chromosome conditions. 2024. https://ranzcog.edu.au/resources/statements-and-guidelines-directory/ [cited 2025 Mar 5]
Publication types
LinkOut - more resources
Full Text Sources