Self-assembly of paclitaxel derivative and fructose as a potent inducer of immunogenic cell death to enhance cancer immunotherapy
- PMID: 40343162
- PMCID: PMC12059333
- DOI: 10.1016/j.mtbio.2025.101793
Self-assembly of paclitaxel derivative and fructose as a potent inducer of immunogenic cell death to enhance cancer immunotherapy
Abstract
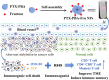
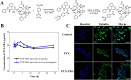
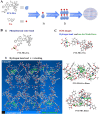
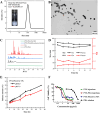
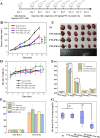
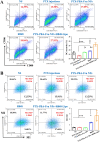
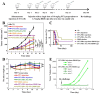
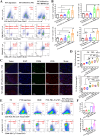
Immunotherapy shows promise for tumor control but is limited by low response rates. Paclitaxel (PTX) induces immunogenic cell death (ICD), yet conventional delivery systems face challenges like low drug loading and insufficient intracellular accumulation, reducing ICD efficacy. Small-molecule self-assembled PTX nanoparticles offer a promising solution due to high drug loading and dose delivery. In this study, PTX was conjugated with phenylboronic acid (PBA) to form the derivative PTX-PBA, which spontaneously self-assembled with fructose into nanoparticles (PTX-PBA-Fru NPs). These nanoparticles exhibited a uniform size of 107.8 ± 2.9 nm, a PDI of 0.064 ± 0.042, and a zeta potential of -12.2 ± 0.9 mV, with spherical morphology. In 4T1 tumor-bearing mice, PTX-PBA-Fru NPs significantly enhanced tumor inhibition (p < 0.001) and increased body weight (p < 0.05). No allergic reactions in healthy Balb/c mice and the maximum tolerated intravenous dose reached 200 mg/kg, underscoring its favorable safety profile of PTX-PBA-Fru NPs. The ICD effects induced by PTX-PBA-Fru NPs, when combined with the immunomodulator resiquimod (R848), elicited a robust anti-tumor immune response. This combination therapy effectively remodeled the immunosuppressive tumor microenvironment and achieved a 37.5 % tumor eradication rate. Moreover, it established long-term immune memory, providing protection against tumor re-challenge. This novel PTX formulation demonstrates strong anti-tumor effects, safety, and clinical potential in combination with R848-based immunotherapy.
Keywords: Chemoimmunotherapy; ICD; Paclitaxel derivative; Phenylboronic acid; Resiquimod; Self-assembly.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Nanomedicine based on chemotherapy-induced immunogenic death combined with immunotherapy to enhance antitumor immunity.Front Pharmacol. 2024 Dec 4;15:1511423. doi: 10.3389/fphar.2024.1511423. eCollection 2024. Front Pharmacol. 2024. PMID: 39697556 Free PMC article.
-
Smart Polymeric Nanoparticles with pH-Responsive and PEG-Detachable Properties (II): Co-Delivery of Paclitaxel and VEGF siRNA for Synergistic Breast Cancer Therapy in Mice.Int J Nanomedicine. 2021 Aug 13;16:5479-5494. doi: 10.2147/IJN.S313339. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34413645 Free PMC article.
-
Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy.Theranostics. 2020 Jul 9;10(18):8382-8399. doi: 10.7150/thno.45391. eCollection 2020. Theranostics. 2020. PMID: 32724476 Free PMC article.
-
Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy.Theranostics. 2021 Feb 20;11(9):4171-4186. doi: 10.7150/thno.42260. eCollection 2021. Theranostics. 2021. PMID: 33754055 Free PMC article.
-
Sequential pH/GSH-responsive stealth nanoparticles for co-delivery of anti-PD-1 antibody and paclitaxel to enhance chemoimmunotherapy of lung cancer.Eur J Med Chem. 2025 Mar 5;285:117273. doi: 10.1016/j.ejmech.2025.117273. Epub 2025 Jan 10. Eur J Med Chem. 2025. PMID: 39813775
References
LinkOut - more resources
Full Text Sources

