A biomimetic nanomedicine alleviates liver transplant-related biliary injury by sequentially inhibiting oxidative stress and regulating macrophage polarization via Nrf-2/HO-1 and JNK pathways
- PMID: 40343167
- PMCID: PMC12059350
- DOI: 10.1016/j.mtbio.2025.101797
A biomimetic nanomedicine alleviates liver transplant-related biliary injury by sequentially inhibiting oxidative stress and regulating macrophage polarization via Nrf-2/HO-1 and JNK pathways
Abstract
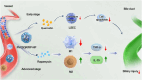
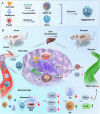
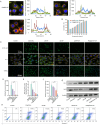
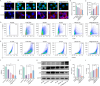
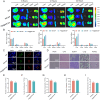
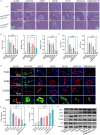
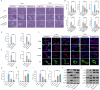
Liver transplantation is an effective method for treating end-stage liver disease. However, 10-20 % of liver transplantation patients develop biliary injury, the main cause of which is ischemia-reperfusion injury (IRI), which consists of oxidative stress injury in the early stage and inflammatory injury in the advanced stage. Biliary injury seriously affects patient outcomes and even leads to mortality, and there are few effective treatments for IRI. Herein, nanoparticles containing quercetin (QR) and rapamycin (RP) coated with poly (lactic-co-glycolic acid) (PLGA) and encapsulated by platelet membrane (PM) were designed to treat IRI in the liver transplantation. The specific binding of ICAM-1 expressed on the PM to integrins (e.g., LFA-1 and Mac-1) in damaged vascular endothelial cells, as well as the interaction between P-selectin on the platelet surface and PSGL-1 on the macrophage surface, allows the accumulation of these biomimetic cell membrane-encapsulated nanoparticles, and subsequently, the delivery of both drugs, to ischemia-reperfusion sites in the liver. The encapsulated QR alleviated oxidative stress injury by activating the Nrf-2/HO-1 signaling pathway in the early stage in model rats with IRI and liver transplantation models. Moreover, RP alleviated inflammatory damage in the advanced stage by suppressing the JNK signaling pathway in M1 macrophages. Thus, these biomimetic nanoparticles that intervene in IRI to alleviate both the early oxidative stress and the advanced inflammatory response constitute a novel delivery system for managing biliary injury after liver transplantation.
Keywords: Biliary injury; Biomimetic nanoparticles; JNK signaling pathway; Liver transplantation; Nrf-2/HO-1 signaling pathway.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protective effect of Saxagliptin on diabetic rats with renal ischemia reperfusion injury by targeting oxidative stress and mitochondrial apoptosis pathway through activating Nrf-2/HO-1 signaling.Transpl Immunol. 2023 Feb;76:101762. doi: 10.1016/j.trim.2022.101762. Epub 2022 Nov 23. Transpl Immunol. 2023. PMID: 36435353
-
Curculigoside mitigates hepatic ischemia/reperfusion-induced oxidative stress, inflammation, and apoptosis via activation of the Nrf-2/HO-1 pathway.Hum Exp Toxicol. 2022 Jan-Dec;41:9603271221087146. doi: 10.1177/09603271221087146. Hum Exp Toxicol. 2022. PMID: 35331031
-
Scutellarin alleviates renal ischemia-reperfusion injury by inhibiting the MAPK pathway and pro-inflammatory macrophage polarization.FASEB J. 2024 Jul 15;38(13):e23769. doi: 10.1096/fj.202302243R. FASEB J. 2024. PMID: 38958951
-
Nanotheranostics for the Management of Hepatic Ischemia-Reperfusion Injury.Small. 2021 Jun;17(23):e2007727. doi: 10.1002/smll.202007727. Epub 2021 Apr 14. Small. 2021. PMID: 33852769 Review.
-
Biomimetic nanoparticles for inflammation targeting.Acta Pharm Sin B. 2018 Jan;8(1):23-33. doi: 10.1016/j.apsb.2017.12.002. Epub 2017 Dec 24. Acta Pharm Sin B. 2018. PMID: 29872620 Free PMC article. Review.
References
-
- Samstein B., Smith A.R., Freise C.E., Zimmerman M.A., Baker T., Olthoff K.M., Fisher R.A., Merion R.M. Complications and their resolution in recipients of deceased and living donor liver transplants: findings from the A2ALL cohort study. Am. J. Transplant. 2016;16:594–602. doi: 10.1111/ajt.13479. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

