Regulated cell death and DAMPs as biomarkers and therapeutic targets in normothermic perfusion of transplant organs. Part 1: their emergence from injuries to the donor organ
- PMID: 40343197
- PMCID: PMC12060192
- DOI: 10.3389/frtra.2025.1571516
Regulated cell death and DAMPs as biomarkers and therapeutic targets in normothermic perfusion of transplant organs. Part 1: their emergence from injuries to the donor organ
Abstract
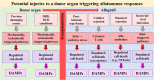
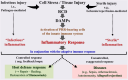
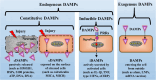
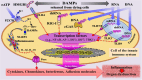
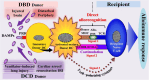
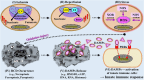
This Part 1 of a bipartite review commences with a succinct exposition of innate alloimmunity in light of the danger/injury hypothesis in Immunology. The model posits that an alloimmune response, along with the presentation of alloantigens, is driven by DAMPs released from various forms of regulated cell death (RCD) induced by any severe injury to the donor or the donor organ, respectively. To provide a strong foundation for this review, which examines RCD and DAMPs as biomarkers and therapeutic targets in normothermic regional perfusion (NRP) and normothermic machine perfusion (NMP) to improve outcomes in organ transplantation, key insights are presented on the nature, classification, and functions of DAMPs, as well as the signaling mechanisms of RCD pathways, including ferroptosis, necroptosis, pyroptosis, and NETosis. Subsequently, a comprehensive discussion is provided on major periods of injuries to the donor or donor organs that are associated with the induction of RCD and DAMPs and precede the onset of the innate alloimmune response in recipients. These periods of injury to donor organs include conditions associated with donation after brain death (DBD) and donation after circulatory death (DCD). Particular emphasis in this discussion is placed on the different origins of RCD-associated DAMPs in DBD and DCD and the different routes they use within the circulatory system to reach potential allografts. The review ends by addressing another particularly critical period of injury to donor organs: their postischemic reperfusion following implantation into the recipient-a decisive factor in determining transplantation outcome. Here, the discussion focuses on mechanisms of ischemia-induced oxidative injury that causes RCD and generates DAMPs, which initiate a robust innate alloimmune response.
Keywords: DAMPs; donation after brain death; donation after circulatory death; injuries to donor organs; innate alloimmunity; normothermic machine perfusion; normothermic regional perfusion; regulated cell death.
© 2025 Land and Linkermann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Land WG. Injury to allografts: innate immune pathways to acute and chronic rejection. Saudi J Kidney Dis Transpl. (2005) 16:520–39. Available online at: https://pubmed.ncbi.nlm.nih.gov/18202507/. - PubMed
-
- Land WG. Innate Alloimmunity: Part 2: Innate Immunity and Allograft Rejection. Lengerich: Baskent University, Ankara, Pabst Science Publishers; (2011). p. 760. Available online at: https://www.pabst-publishers.com/shop-checkout/suche/suchedetailergb?tt_...
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

