Post-symptomatic NLRP3 inhibition rescues cognitive impairment and mitigates amyloid and tau driven neurodegeneration
- PMID: 40343261
- PMCID: PMC12055592
- DOI: 10.1038/s44400-025-00011-5
Post-symptomatic NLRP3 inhibition rescues cognitive impairment and mitigates amyloid and tau driven neurodegeneration
Abstract
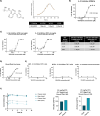
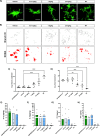
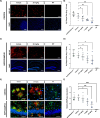
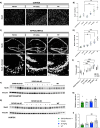
Emerging evidence has established neuroinflammation as a primary driver of progressive neuronal loss observed across neurodegenerative diseases (NDDs). The NLRP3 inflammasome is a cytosolic immunoprotective danger sensing complex, which when aberrantly activated drives neuroinflammation, propagates amyloid deposition, and neurodegeneration. Herein, we report the therapeutic benefit of NLRP3 inflammasome inhibition in Alzheimer's disease (AD), using a novel and selective brain-penetrant small molecule NLRP3 inhibitor, VEN-02XX, which we profiled in the 5XFAD/Rubicon KO AD model. We demonstrate for the first time that targeting NLRP3, post-symptomatic establishment, rescues cognitive deficits, mitigates neuronal loss, and is sufficient to significantly reduce reactive microgliosis, neuroinflammation and tau pathology. Our data further suggest that pharmacological inhibition of NLRP3, after disease onset, has the potential to reduce cortical and hippocampal amyloid burden. Together, these results highlight the potential for NLRP3 inhibition as a symptomatic and disease modifying therapeutic target for AD pathology and more broadly NDDs.
Keywords: Drug discovery; Immunology; Neurodegeneration; Neurodegenerative diseases; Neuroscience.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsA.A., R.F., R.S., M.-A.G. and A.C. declare they are employees of Ventus Therapeutics, Inc., based in Montreal, Canada, and M.A.C. is an employee of Ventus Therapeutics U.S., Inc., based in Waltham, Massachusetts, USA. M.R. was a former employee at Ventus Therapeutics, Inc., based in Montreal, Canada. Ventus Therapeutics Inc. is a subsidiary of Ventus Therapeutics U.S., Inc., collectively referred to herein as “Ventus”. B.L.H. consults for Ventus and is the co-founder and CSO of Asha Therapeutics.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
