Decoding elegant interplay among different stereo-electronic effects due to the ancient prolyl-4-hydroxylation stabilizing collagenous helicity
- PMID: 40343281
- PMCID: PMC12059709
- DOI: 10.1016/j.isci.2025.112393
Decoding elegant interplay among different stereo-electronic effects due to the ancient prolyl-4-hydroxylation stabilizing collagenous helicity
Abstract
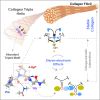
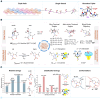
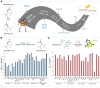
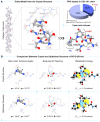
Prolyl-4-hydroxylation is an ancient evolutionarily conserved post-translational modification (PTM) critical for both structural and regulatory functions in multicellular life forms. This PTM plays a pivotal role in stabilizing collagen's triple helix by influencing the puckering of the pyrrolidine ring. The elegant interplay between ring pucker, torsional angles, peptide bond isomerization, and charge-transfer interactions (O···C=O n→π∗ and σ→σ∗) attaining the helical stability remains underappreciated. Using density functional theory calibrated against gold standard ab initio methods, we analyzed a physiologically relevant collagenous peptide proline-4-hydroxyproline-glycine (PO4G) to establish the correlation between stereo-electronic effects due to prolyl-4-hydroxylation. Our results show that 4(R)-hydroxylation promotes an exo ring pucker, optimizing main-chain torsional angles for a stable trans peptide bond and maximizing the n→π∗ interaction (E n→π∗ = 0.9 kcal/mol) by tuning Bürgi-Dunitz trajectory, and maximizes σ→σ∗ interactions between axial C-H σ-electrons and C-OH∗ orbitals of the pyrrolidine ring. This study reveals the intricate stereo-electronic effects driving collagen's structural stability.
Keywords: Physical chemistry; Quantum chemical calculations; Quantum chemistry.
© 2025 The Author(s).
Conflict of interest statement
There are no competing interests.
Figures



Similar articles
-
Collagen stability: insights from NMR spectroscopic and hybrid density functional computational investigations of the effect of electronegative substituents on prolyl ring conformations.J Am Chem Soc. 2002 Mar 20;124(11):2497-505. doi: 10.1021/ja0166904. J Am Chem Soc. 2002. PMID: 11890798
-
Intramolecular hydrogen bond-controlled prolyl amide isomerization in glucosyl 3'(S)-hydroxy-5'-hydroxymethylproline hybrids: influence of a C-5'-hydroxymethyl substituent on the thermodynamics and kinetics of prolyl amide cis/trans isomerization.J Org Chem. 2009 May 15;74(10):3735-43. doi: 10.1021/jo9003458. J Org Chem. 2009. PMID: 19354261
-
Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms.J Biol Chem. 2018 May 18;293(20):7645-7658. doi: 10.1074/jbc.RA118.002200. Epub 2018 Apr 3. J Biol Chem. 2018. PMID: 29615493 Free PMC article.
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
-
Structural aspects of hydroxyproline-containing proteins.J Biomol Struct Dyn. 1983 Dec;1(3):843-55. doi: 10.1080/07391102.1983.10507485. J Biomol Struct Dyn. 1983. PMID: 6401122 Review.
References
LinkOut - more resources
Full Text Sources

