Multifaceted profiling of virus-specific CD8 T cells reveals distinct immune signatures against cytomegalovirus infection states during pregnancy
- PMID: 40343282
- PMCID: PMC12059710
- DOI: 10.1016/j.isci.2025.112416
Multifaceted profiling of virus-specific CD8 T cells reveals distinct immune signatures against cytomegalovirus infection states during pregnancy
Abstract
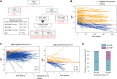
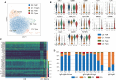
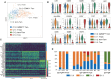
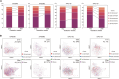
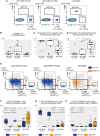
Anti-cytomegalovirus (CMV) serological testing, including the IgG avidity index (AI), is used to assess CMV infection phases during pregnancy. However, little is known about anti-CMV cellular immunity during pregnancy, particularly its relation to serological diagnosis. Herein, using MHC-dextramer single-cell RNA sequencing and flow cytometry, we characterized IE1 and pp65 CMV-antigen specific CD8 T cells from pregnant women with different anti-CMV serological patterns, including IgG+IgM+/AI-low, IgG+IgM+/AI-high, and IgG+IgM-. In IgG+IgM+/AI-low and IgG+IgM+/AI-high specimens, CMV-specific T cells consisted largely of effectors, with a minor but characteristic proportion of memory T cells, including HLA-DR-positive memory precursors and granzyme K-high memory cells reactive to IE1. Conversely, IgG+IgM- cases had a distinctive expansion of pp65-specific terminally differentiated T effector memory with a signature of convergent clonal selection. Our findings revealed that different CMV infection phases have characteristic patterns of CD8 cell phenotype and antigen recognition, potentially offering a new approach for assessing congenital infection risk.
Keywords: biological sciences; immunology; pregnancy.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rawlinson W.D., Boppana S.B., Fowler K.B., Kimberlin D.W., Lazzarotto T., Alain S., Daly K., Doutré S., Gibson L., Giles M.L., et al. Congenital Cytomegalovirus Infection in Pregnancy and the Neonate: Consensus Recommendations for Prevention, Diagnosis, and Therapy. Lancet Infect. Dis. 2017;17:e177–e188. doi: 10.1016/S1473-3099(17)30143-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

