Synthesis, molecular docking, and in vitro activity of a novel angiotensin-converting enzyme 2 inhibitor, LMS1007: a potential molecule in Covid-19 and cancer treatments
- PMID: 40343305
- PMCID: PMC12060149
- DOI: 10.1039/d5ra01134e
Synthesis, molecular docking, and in vitro activity of a novel angiotensin-converting enzyme 2 inhibitor, LMS1007: a potential molecule in Covid-19 and cancer treatments
Abstract
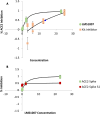
Angiotensin-converting enzyme 2 (ACE2) is a validated commonly studied in the pathology of several diseases, including novel coronavirus and breast cancer. Herein, we report the synthesis, molecular docking, and validation of a novel ACE2 inhibitor that was previously discovered by our team based on diverse scaffolds of other ACE2 inhibitors and carnosine. The synthesized 4-subsitituted imidazole compound, namely, LMS1007, was characterized through 1H-NMR, LC-MS, and SFC. LMS1007 was then tested in vitro with ACE2 and viral spike protein-ACE2 inhibitor kits and was found to be approximately 100 times more potent as an ACE2 inhibitor than carnosine. However, it was less potent than the standard ACE2 inhibitor. In the same concentration range of the standard drug for ACE2 inhibition, LMS1007 demonstrated similar inhibitory effects on the interaction of the viral spike protein with ACE2. LMS1007 had an inhibitory concentration of 50% (IC50) at a concentration of 2.3 mM in all kits. LMS1007, similar to carnosine in breast cancer cell lines, exhibited potential inhibitory effects on the ACE2-mediated host uptake of Covid-19. Thus, a thorough review and discussion are provided on the role of ACE2 as an attractive target for the development of new drugs for Covid-19 treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures
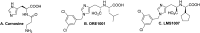




Similar articles
-
Carnosine to Combat Novel Coronavirus (nCoV): Molecular Docking and Modeling to Cocrystallized Host Angiotensin-Converting Enzyme 2 (ACE2) and Viral Spike Protein.Molecules. 2020 Nov 28;25(23):5605. doi: 10.3390/molecules25235605. Molecules. 2020. PMID: 33260592 Free PMC article.
-
Deciphering angiotensin converting enzyme 2 (ACE2) inhibition dynamics: Carnosine's modulatory role in breast cancer proliferation - A clinical sciences perspective.Heliyon. 2024 Sep 29;10(19):e38685. doi: 10.1016/j.heliyon.2024.e38685. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39398078 Free PMC article.
-
Multidisciplinary Approaches Identify Compounds that Bind to Human ACE2 or SARS-CoV-2 Spike Protein as Candidates to Block SARS-CoV-2-ACE2 Receptor Interactions.mBio. 2021 Mar 30;12(2):e03681-20. doi: 10.1128/mBio.03681-20. mBio. 2021. PMID: 33785634 Free PMC article.
-
Angiotensin-converting enzyme 2, coronavirus disease 2019, and abdominal aortic aneurysms.J Vasc Surg. 2021 Nov;74(5):1740-1751. doi: 10.1016/j.jvs.2021.01.051. Epub 2021 Feb 15. J Vasc Surg. 2021. PMID: 33600934 Free PMC article. Review.
-
Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5. J Med Virol. 2020. PMID: 32221983 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

