Protein-based nanoparticles for antimicrobial and cancer therapy: implications for public health
- PMID: 40343307
- PMCID: PMC12060137
- DOI: 10.1039/d5ra01427a
Protein-based nanoparticles for antimicrobial and cancer therapy: implications for public health
Abstract
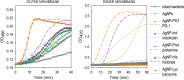
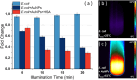
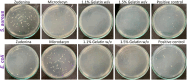
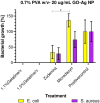
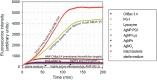
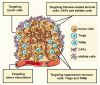
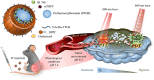
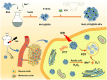
This review discusses the growing potential of protein-based nanoparticles (PBNPs) in antimicrobial and cancer therapies, emphasizing their mechanisms of action, applications, and future prospects. In antimicrobial therapy, PBNPs exhibit several mechanisms of action, including disruption of microbial membranes, enhanced antibiotic delivery, immune modulation, and biofilm disruption. Protein nanoparticles like albumin, lactoferrin, gelatin, and peptide-based variants enhance the efficacy of antibiotics, offering targeted approaches to combat multidrug-resistant pathogens. Their ability to improve drug localization and enhance microbial eradication represents a significant advancement in infectious disease management. In cancer therapy, PBNPs facilitate targeted drug delivery, controlled release, tumor microenvironment modulation, and photothermal and photodynamic therapies. Nanoparticles such as Abraxane® and engineered ferritin nanocages are at the forefront of cancer treatment, enhancing the precision and effectiveness of chemotherapy while minimizing adverse effects. Additionally, silk fibroin nanoparticles are being explored for their biodegradability and targeting capabilities. Despite their promise, challenges remain, including the scalability of production, long-term safety concerns, regulatory approval processes, and environmental impact. Addressing these issues through rigorous research and innovation is crucial for integrating PBNPs into mainstream therapeutic practices. PBNPs offer transformative solutions in both antimicrobial and cancer therapies, with significant implications for improving public health outcomes globally.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures









References
-
- Egwonor L. I. Awoyemi R. F. Owolabi B. Aworinde O. R. Kajola R. O. Hazeez A. et al., Cutting-edge developments in zinc oxide nanoparticles: Synthesis and applications for enhanced antimicrobial and UV protection in healthcare solutions. RSC Adv. 2024;14:20992–21034. doi: 10.1039/D4RA02452D. - DOI - PMC - PubMed
-
- Maliki M., Omorogbe S. O., et al., Incisive review on magnetic iron oxide nanoparticles and their use in the treatment of bacterial infections, in TMS 2023 152nd Annual Meeting & Exhibition Supplemental Proceedings, Cham, Springer, 2023, 10.1007/978-3-031-22524-6_44 - DOI
Publication types
LinkOut - more resources
Full Text Sources

