Black is the new orange: inline synthesis of silica-coated iron oxide nanoparticles produced via gas-phase in a matrix burner
- PMID: 40343314
- PMCID: PMC12059999
- DOI: 10.1039/d5ra00808e
Black is the new orange: inline synthesis of silica-coated iron oxide nanoparticles produced via gas-phase in a matrix burner
Abstract
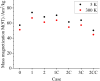
Superparamagnetic iron oxide nanoparticles (IONPs) have a large range of applications, such as pollutant removal and inductive heating. Some of these applications benefit from coating the IONPs with silica (SiO2) to conserve their properties and/or prevent their aggregation; yet, the habitual synthesis methodologies require several steps, which limit their industrial scalability. In this work, we explore the capability to synthesize and stabilize oxidation-sensitive phases of IONPs via gas-phase flame synthesis as an alternative methodology that enables continuous operation. The addition of an inline quench gas nozzle-to avoid aggregation/agglomeration-and a coating nozzle is investigated to clarify their roles in contributing to the properties of the resultant coated IONPs. Three different quench and coating configuration heights above burner (HAB) are studied. The resultant synthesized Fe x O y |SiO2 core-shell nanoparticles are characterized using (scanning) transmission electron microscopy ((S)TEM), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), elemental analysis, dynamic light scattering (DLS), Mössbauer spectroscopy, magnetometry, and energy-dispersive X-ray spectroscopy (EDX) from scanning electron microscopy (SEM). Results show that the synthesized nanoparticles presented a mixture of oxidation states-mainly magnetite (Fe3O4) and maghemite (γ-Fe2O3) phases-and a narrow primary particle size distribution. Quenching the IONPs early decreased the nanoparticle agglomeration/aggregation up to one order of magnitude. Moreover, homogeneous coating was achieved in all cases. Increasing the coating thickness helped reduce oxygen diffusion to the iron oxide core of the coated IONPs, conserving more magnetite phase in the coated IONP cores. These insights allowed us to conclude that targeted coated IONPs can be successfully produced through gas-phase synthesis using a flame reactor. In the near future, the long-term stability of IONP properties will be explored using this inline coating.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater.Nanomaterials (Basel). 2020 Aug 7;10(8):1551. doi: 10.3390/nano10081551. Nanomaterials (Basel). 2020. PMID: 32784715 Free PMC article.
-
Papain grafted into the silica coated iron-based magnetic nanoparticles 'IONPs@SiO2-PPN' as a new delivery vehicle to the HeLa cells.Nanotechnology. 2020 May 8;31(19):195603. doi: 10.1088/1361-6528/ab6fd4. Epub 2020 Jan 24. Nanotechnology. 2020. PMID: 31978907
-
Synthesis, characterization and antibacterial activity of superparamagnetic nanoparticles modified with glycol chitosan.Sci Technol Adv Mater. 2012 Feb 2;13(1):015002. doi: 10.1088/1468-6996/13/1/015002. eCollection 2012 Feb. Sci Technol Adv Mater. 2012. PMID: 27877469 Free PMC article.
-
Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future.Materials (Basel). 2020 Oct 18;13(20):4644. doi: 10.3390/ma13204644. Materials (Basel). 2020. PMID: 33080937 Free PMC article. Review.
-
Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review.Anal Chim Acta. 2013 Jul 30;789:1-16. doi: 10.1016/j.aca.2013.04.021. Epub 2013 Apr 29. Anal Chim Acta. 2013. PMID: 23856225 Review.
References
-
- Voss L. Hsiao I. L. Ebisch M. Vidmar J. Dreiack N. Bohmert L. Stock V. Braeuning A. Loeschner K. Laux P. Thunemann A. F. Lampen A. Sieg H. Food Chem. 2020;327:127000. - PubMed
-
- Saharan P. Chaudhary G. R. Mehta S. K. Umar A. J. Nanosci. Nanotechnol. 2014;14:627–643. - PubMed
-
- Kumar P. Tomar V. Kumar D. Joshi R. K. Nemiwal M. Tetrahedron. 2022:106–107.
-
- Hammad M. Hardt S. Mues B. Salamon S. Landers J. Slabu I. Wende H. Schulz C. Wiggers H. J. Alloys Compd. 2020;824:153814.
-
- Teleki A. Haufe F. L. Hirt A. M. Pratsinis S. E. Sotiriou G. A. RSC Adv. 2016;6:21503–21510.
LinkOut - more resources
Full Text Sources

