Electron donor-acceptor complex-driven photocatalyst-free synthesis of nitrocyclopropanes
- PMID: 40343315
- PMCID: PMC12060227
- DOI: 10.1039/d5ra02540k
Electron donor-acceptor complex-driven photocatalyst-free synthesis of nitrocyclopropanes
Abstract
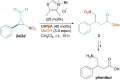
Herein, a visible light-promoted metal-free protocol for the synthesis of nitrocyclopropanes under mild conditions is reported. Specifically, the process is driven by the photochemical activity of ternary EDA complexes formed upon complexation of α-bromonitrostyrenes and DIPEA in the presence of benzaldehyde. This reaction provides a variety of densely functionalized cyclopropanes with good selectivity under mild reaction conditions. Mechanistic investigations on the aspects of the process also demonstrate formation of the hypothesized EDA complex.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Direct C2-H alkylation of indoles driven by the photochemical activity of halogen-bonded complexes.Beilstein J Org Chem. 2023 Apr 27;19:575-581. doi: 10.3762/bjoc.19.42. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37153645 Free PMC article.
-
Electron Donor-Acceptor Complex Driven Photocatalyst-Free Trifluoromethylation of Heterocycles.Chemistry. 2024 Jun 3;30(31):e202400237. doi: 10.1002/chem.202400237. Epub 2024 Apr 23. Chemistry. 2024. PMID: 38556465
-
Visible-Light-Promoted and Electron Donor-Acceptor Complex-Driven Double Csp2-H Bond Functionalization of 2-Arylindoles: A Strategy for the Synthesis of Benzo[a]carbazoles.Org Lett. 2024 Sep 13;26(36):7614-7619. doi: 10.1021/acs.orglett.4c02723. Epub 2024 Sep 5. Org Lett. 2024. PMID: 39235141
-
N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C-H Functionalization via Radical-Based Processes under Visible Light Irradiation.Acc Chem Res. 2022 Oct 18;55(20):3043-3056. doi: 10.1021/acs.accounts.2c00530. Epub 2022 Sep 27. Acc Chem Res. 2022. PMID: 36166489 Review.
-
Synthetic reactions driven by electron-donor-acceptor (EDA) complexes.Beilstein J Org Chem. 2021 Apr 6;17:771-799. doi: 10.3762/bjoc.17.67. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33889219 Free PMC article. Review.
References
-
- Časar Z. Synthesis. 2020:1315.
- Talele T. T. J. Med. Chem. 2016;59:8712. - PubMed
-
- Ebne C. Carreira E. M. Chem. Rev. 2017;117:11651. - PubMed
- Reissig H.-U. Zimmer R. Chem. Rev. 2003;103:1151. - PubMed
- Carson C. A. Kerr M. A. Chem. Soc. Rev. 2009;38:3051. - PubMed
- Apel C. Christmann M. Tetrahedron. 2021;82:131760.
- Gabbey A. L. Scotchburn K. Rousseaux S. A. L. Nat. Rev. Chem. 2023;7:548. - PubMed
-
- Wu W. Lin Z. Jiang H. Org. Biomol. Chem. 2018;16:7315. - PubMed
- Mato M. Franchino A. García-Morales C. Echavarren A. Chem. Rev. 2021;121:8613. - PMC - PubMed
- Nam D. Steck V. Potenzino R. J. Fasan R. J. Am. Chem. Soc. 2021;143:2221. - PMC - PubMed
- Johnson J. D. Teeples C. R. Akkawi N. R. Wilkerson-Hill S. M. J. Am. Chem. Soc. 2022;144:14471. - PubMed
- Crompton J. L. Frost J. R. Rowe S. M. Christensen K. E. Donohoe T. J. Org. Lett. 2023;25:5253. - PMC - PubMed
- Sansinenea E. Ortíz A. Eur. J. Org Chem. 2022:e202200210.
- Kim M. J. Wang D. J. Targos K. García U. A. Harris A. F. Guzei I. A. Wickens Z. K. Angew. Chem., Int. Ed. 2023;62:e202303032. - PMC - PubMed
- Seavill P. W. Nat. Synth. 2024;3:280.
- Menzek A. Sirtbasi S. Sahin E. Bayrak C. Olgun M. E. Yavari A. Bayram S. Rezaei M. J. Mol. Struct. 2025;1324:140807.
-
- Simmons H. E. Smith R. D. J. Am. Chem. Soc. 1958;80:5323.
- Simmons H. E. Smith R. D. J. Am. Chem. Soc. 1959;81:4256.
- Concellón J. M. Rodríguez-Solla H. Concellón C. del Amo V. Chem. Soc. Rev. 2010;39:4103. - PubMed
- Rodríguez-Solla H. Concellón C. del Amo V. Díaz-Pardo A. Blanco E. G. García-Granda S. Díaz M. R. Llavona R. Soengas R. G. Eur. J. Org Chem. 2013:4953.
LinkOut - more resources
Full Text Sources