This is a preprint.
Neural Networks of the Mouse Primary Visceromotor Cortex
- PMID: 40343331
- PMCID: PMC12060971
- DOI: 10.21203/rs.3.rs-4125909/v1
Neural Networks of the Mouse Primary Visceromotor Cortex
Update in
-
Neural networks of the mouse visceromotor cortex.Nature. 2025 Aug 27. doi: 10.1038/s41586-025-09360-w. Online ahead of print. Nature. 2025. PMID: 40866707
Abstract
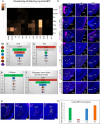
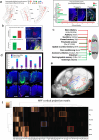
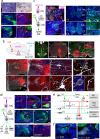
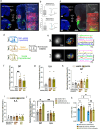
The medial prefrontal cortex (MPF) regulates emotions, stress responses, and goal-directed behaviors like attention and decision-making. However, the precise mechanisms underlying MPF function remain poorly understood, largely due to an incomplete characterization of its neural circuitry. Leveraging neuroanatomical, neurophysiological, and behavioral techniques, we present a detailed wiring diagram of the MPF, with a particular focus on the dorsal peduncular area (DP), an underexplored MPF area implicated in psychological stress, fear conditioning, anxiety, depression, and opioid addiction. Our analysis identifies the deep (DPd) and superficial (DPs) layers of the DP, together with the infralimbic area (ILA), as key components of the primary visceromotor cortex, that generate monosynaptic projections to regulate neuroendocrine, sympathetic, and parasympathetic functions in distinct, yet coordinated ways. Further, we demonstrate that the DP serves as a unique network hub for unidirectional cortical information flow, that integrates diverse cortical inputs and modulates social behavior. Based on the mesoscale connectome of entire MPF, we propose a unified MPF network model that regulates different aspects of motor actions associated with goal-directed behavior. This study provides novel insights into the complex role of the MPF in orchestrating physiological and behavioral responses to environmental stimuli in mammals.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures



References
-
- Fuster J. The Prefrontal Cortex. Fifth edn, (Elsevier, 2015).
Methods References:
Publication types
Grants and funding
- P50 HD103557/HD/NICHD NIH HHS/United States
- R01 MH130941/MH/NIMH NIH HHS/United States
- R01 DC008983/DC/NIDCD NIH HHS/United States
- RF1 MH128888/MH/NIMH NIH HHS/United States
- R01 NS113124/NS/NINDS NIH HHS/United States
- RF1 MH114112/MH/NIMH NIH HHS/United States
- R01 MH132736/MH/NIMH NIH HHS/United States
- UF1 NS122124/NS/NINDS NIH HHS/United States
- R01 MH094360/MH/NIMH NIH HHS/United States
- U01 MH114829/MH/NIMH NIH HHS/United States
- RF1 NS132912/NS/NINDS NIH HHS/United States
- R01 NS133744/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

