This is a preprint.
Epithelial-mesenchymal cell competition coordinates fate transitions across tissue compartments during lung development and fibrosis
- PMID: 40343336
- PMCID: PMC12060972
- DOI: 10.21203/rs.3.rs-6189965/v1
Epithelial-mesenchymal cell competition coordinates fate transitions across tissue compartments during lung development and fibrosis
Abstract
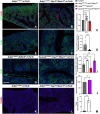
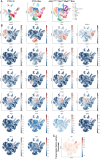
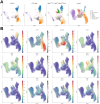
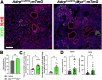
Morphogenesis and cell state transitions must be coordinated in time and space to produce a functional tissue. In this study, we reveal that lung mesenchymal Yap levels and fitness antagonize epithelial Yap levels and stemness during lung development and repair following bleomycin injury. Elevated mesenchymal Yap signaling and fitness antagonize epithelial Yap levels and stemness, accelerating alveolar epithelial differentiation while impairing branching during lung development or bronchiolization after bleomycin injury. Conversely, mesenchymal Snail/Slug sequesters Yap/Taz to direct an adipogenic differentiation program towards alveolar fibroblast 1 (AF1) during both lung development and the resolution of pulmonary fibrosis. On the other hand, Yap/Myc-Tead binding instructs a myogenic differentiation program. Through our experiments and modeling, we identify tissue-scale mechanical cooperation as a pivotal factor in orchestrating organ formation and regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis.Elife. 2023 May 11;12:e85092. doi: 10.7554/eLife.85092. Elife. 2023. PMID: 37166104 Free PMC article.
-
Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation.Cell Cycle. 2017 Mar 4;16(5):399-405. doi: 10.1080/15384101.2017.1280643. Epub 2017 Jan 23. Cell Cycle. 2017. PMID: 28112996 Free PMC article.
-
Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury.JCI Insight. 2023 Sep 7;8(19):e173374. doi: 10.1172/jci.insight.173374. JCI Insight. 2023. PMID: 37676731 Free PMC article.
-
YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis.Cells. 2024 Sep 10;13(18):1519. doi: 10.3390/cells13181519. Cells. 2024. PMID: 39329703 Free PMC article. Review.
-
The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury.Front Cell Dev Biol. 2022 Jul 19;10:894737. doi: 10.3389/fcell.2022.894737. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35927987 Free PMC article. Review.
References
-
- Volckaert T. et al. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 140, 3731–3742 (2013). https://doi.org:dev.096560 [pii] 10.1242/dev.096560 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

