This is a preprint.
Cryo-EM reveals remodeling of a tandem riboswitch at 2.9 Å resolution
- PMID: 40343338
- PMCID: PMC12060979
- DOI: 10.21203/rs.3.rs-6422592/v1
Cryo-EM reveals remodeling of a tandem riboswitch at 2.9 Å resolution
Abstract
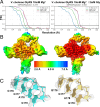
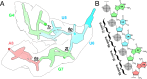
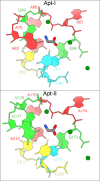
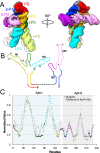
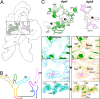
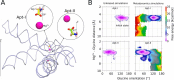
Riboswitches are non-coding RNA sequences that control cellular processes through ligand binding. Conformational heterogeneity is fundamental to riboswitch functionality, yet this same attribute makes structural characterization of these mRNA elements challenging. Here, we use a combination of molecular dynamics and cryo-electron microscopy to expound the flexible nature of the glycine riboswitch tandem aptamers and characterize diMerent structural populations. We find that Mg2+ partially stabilizes the fully folded state, resulting in one-third of the particles adopting a unique "walking man" conformation, consisting of a rigidified core and two dynamic helices, and two-thirds adopting distinct, partially folded states. Glycine interactions double the relative population of fully folded particles by stabilizing a conserved inter-aptamer Hoogsteen base pair, enabling our capture of a 2.9 Å structure for this RNA-only system. The population data show that glycine and Mg2+ operate synergistically: glycine enhances Mg2+ occupancy, while Mg2+ drives glycine specificity. Our findings indicate that cryo-electron microscopy oMers a promising avenue to characterize RNA folding ensembles.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

