Cardiac Adverse Events in Patients Receiving Immune Checkpoint Inhibitors in the Adjuvant Setting: An FDA Pooled Analysis
- PMID: 40343390
- PMCID: PMC12059289
- DOI: 10.1111/anec.70087
Cardiac Adverse Events in Patients Receiving Immune Checkpoint Inhibitors in the Adjuvant Setting: An FDA Pooled Analysis
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment. By releasing blocks (checkpoints) on the immune system, they elicit powerful antitumor effects. Despite improving survival, ICIs are associated with serious cardiac toxicities. Previous reports have focused on advanced cancer; cardiotoxicity data are therefore limited in the curative setting. We evaluated ICI cardiotoxicity in the non-metastatic setting, where long-term cardiac safety is a growing public health concern.
Methods: ICIs approved in the adjuvant setting were pooled and trials with combination chemotherapy were excluded. Cardiac adverse events (AEs) and emerging cardio-metabolic risks (hyperglycemia, weight gain, hypothyroidism) were assessed. The relative risk (RR) of cardiotoxicity was assessed.
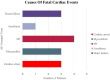
Results: Ten randomized controlled trials of atezolizumab, ipilimumab, nivolumab, and pembrolizumab in multiple solid tumors were evaluated; among 9244 patients, 5338 received ICIs. No trial performed routine cardiac monitoring. Six percent of ICI patients vs. 4.6% in placebo (RR 1.24, 95% CI 1.04, 1.49) had a cardiac AE and 13 (0.2%) of ICI patients experienced a fatal cardiac AE (RR 4.76, 95% CI 1.07, 21.06). Older age and male sex were associated with a higher risk for cardiac fatality. Arrhythmia was the most common cardiac AE; hypothyroidism was more frequent (14% vs. 2.5%) among ICI-treated patients.
Conclusion: This is the largest pooled analysis of cardiac AEs associated with ICIs in the adjuvant setting. Despite no formalized testing for subclinical cardiotoxicity, ICI treatment increased cardiac AEs. These findings are relevant for long-term cancer survivors, clinicians, and particularly in new drug development, where cardiotoxicity may be substantially underestimated.
Keywords: adjuvant; cancer; cardio oncology; cardiotoxicity; immune checkpoint inhibitors; immunotherapy.
© 2025 The Author(s). Annals of Noninvasive Electrocardiology published by Wiley Periodicals LLC.
Conflict of interest statement
Asma Dilawari‐Reimagine Care Inc. Medical oncology consultant until March 2023, River Fall Associates LLC, Integrative oncology practice. Mori Krantz‐President of Colorado American College of Cardiology. None of the other authors listed have any disclosures.
The authors declare no conflicts of interest.
Figures

References
-
- Ascierto, P. A. , Del Vecchio M., Mandala M., et al. 2020. “Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB‐C and Stage IV Melanoma (CheckMate 238): 4‐Year Results From a Multicentre, Double‐Blind, Randomised, Controlled, Phase 3 Trial.” Lancet Oncology 21: 1465–1477. - PubMed
-
- Campbell, P. , Rutten F. H., Lee M. M., Hawkins N. M., and Petrie M. C.. 2024. “Heart Failure With Preserved Ejection Fraction: Everything the Clinician Needs to Know.” Lancet 403: 1083–1092. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

