The diagnostic yield of molecular karyotyping: a retrospective single-center study
- PMID: 40343432
- PMCID: PMC12093118
- DOI: 10.3325/cmj.2025.66.92
The diagnostic yield of molecular karyotyping: a retrospective single-center study
Abstract
Aim: To determine the diagnostic yield of chromosomal microarray analysis (CMA) in different patient groups: intellectual disability and developmental delay (ID/DD), multiple congenital anomalies (MCA), epilepsy, autism spectrum disorder (ASD), reproductive abnormalities, and dysmorphic features.
Methods: We retrospectively reviewed microarray data of 176 patients admitted to the Medical Genetics Outpatient Clinic of Necmettin Erbakan University Medical Faculty Hospital from 2016 to 2022. After the copy number variation (CNV) interpretation, we evaluated the diagnostic strength of CMA in each group.
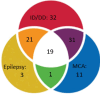
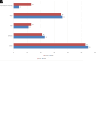
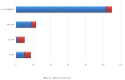
Results: Phenotype-associated CNVs were detected in 20.3% (22/108) of patients with ID/DD, 23.9% (17/71) of patients with MCA, 15.9% of patients (7/44) with epilepsy, 16.6% (4/24) of patients with ASD, and 11.7% (2/17) of those with reproductive abnormalities. Chromosomal gains or losses were found in 43% (35/80) of patients with dysmorphic findings.
Conclusion: This study confirmed the remarkable diagnostic yield of CMA in ID/DD, MCA, and ASD patients, and expanded its value for cases with epilepsy and dysmorphism.
Figures
Similar articles
-
Chromosomal Aberrations in Pediatric Patients with Developmental Delay/Intellectual Disability: A Single-Center Clinical Investigation.Biomed Res Int. 2019 Nov 6;2019:9352581. doi: 10.1155/2019/9352581. eCollection 2019. Biomed Res Int. 2019. PMID: 31781653 Free PMC article.
-
Confirmation of chromosomal microarray as a first-tier clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features.Eur J Paediatr Neurol. 2013 Nov;17(6):589-99. doi: 10.1016/j.ejpn.2013.04.010. Epub 2013 May 24. Eur J Paediatr Neurol. 2013. PMID: 23711909
-
Diagnostic yield of the chromosomal microarray analysis in turkish patients with unexplained development delay/ıntellectual disability(ID), autism spectrum disorders and/or multiple congenital anomalies and new clinical findings.Mol Biol Rep. 2024 Apr 25;51(1):577. doi: 10.1007/s11033-024-09545-y. Mol Biol Rep. 2024. PMID: 38664339
-
Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies.Am J Hum Genet. 2010 May 14;86(5):749-64. doi: 10.1016/j.ajhg.2010.04.006. Am J Hum Genet. 2010. PMID: 20466091 Free PMC article. Review.
-
Experience of chromosomal microarray applied in prenatal and postnatal settings in Hong Kong.Am J Med Genet C Semin Med Genet. 2019 Jun;181(2):196-207. doi: 10.1002/ajmg.c.31697. Epub 2019 Mar 23. Am J Med Genet C Semin Med Genet. 2019. PMID: 30903683 Review.
References
-
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am J Hum Genet. 2010;86:749–64. doi: 10.1016/j.ajhg.2010.04.006. - DOI - PMC - PubMed