Establishment and characterization of Cri Du Chat neuronal stem cells: a novel promising resource to study the syndrome
- PMID: 40343585
- PMCID: PMC12064636
- DOI: 10.1007/s13577-025-01230-x
Establishment and characterization of Cri Du Chat neuronal stem cells: a novel promising resource to study the syndrome
Abstract
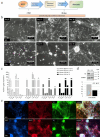
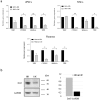
The Cri Du Chat (CdC) Syndrome is a rare chromosome disease condition resulting from variable size deletion occurring on the short arm of one of the chromosomes 5. This disorder, which affects one in 50,000 births, is responsible for developmental retardation, the mechanism of which has remained unexplained. TERT, SEMA5 A, CTNND2, TPPP, mapped in chromosome 5 short arm, are known to be expressed in the brain, and to play a role in the development of the nervous system, oligodentrocytes and in the regulation of glutamatergic and dopaminergic synaptic transmission. It is critical to understand how their haploinsufficiency might affect the development and presentation of the disease. In the absence of an animal model and of significant accessible, human tissue, human pluripotent stem cells (iPSC) directly reprogrammed from patient somatic cells open a new area of disease modeling as they can virtually be differentiated into any cell type. Our study reports, for the first time, the generation of neuronal stem cells (NSCs) from CdC-iPSCs line and in addition, subsequent differentiation into a heterogeneous population of neurons. Gene expression of the mentioned and single copy deleted genes was also evaluated by comparing their expression level in iPSC, NSCs and neuron lines. The present research represents the first and the most innovative approach, to create an in vitro CdC neuronal model to have a new translational framework to study the pathologic processes.
Keywords: Cri du Chat neurons; Cri du Chat syndrome; Deletion chromosome 5p; Disease modeling; IPSCs-NSC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no known competing financial interests. Ethical approval: This study was approved by the A.B.C scientific committee. Informed consent: Not pertinent.
Figures


References
-
- Lejeune J, Lafourcade J, Berger R, Vialatte J, Boeswillwald M, Seringe P. Turpin, R [3 cases of partial deletion of the short arm of a 5 chromosome.]. C R Hebd Seances Acad Sci. 1963;257:3098–102. - PubMed
-
- Nevado J, Bel-Fenellós C, Sandoval-Talamantes AK, Hernández A, Biencinto-López C, Martínez-Fernández ML, Barrúz P, Santos-Simarro F, Mori-Álvarez MÁ, Mansilla E, García-Santiago FA, Valcorba I, Sáenz-Rico B, Martínez-Frías ML, Lapunzina P. Phenotyping and genetic characterization of a cohort of 70 individuals with 5p minus syndrome. Front Genet. 2021;12:645595. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

