Cerebral Amyloid Angiopathy and Downstream Alzheimer Disease Plasma Biomarkers
- PMID: 40343697
- PMCID: PMC12065043
- DOI: 10.1001/jamanetworkopen.2025.8842
Cerebral Amyloid Angiopathy and Downstream Alzheimer Disease Plasma Biomarkers
Abstract
Importance: As amyloid-targeted therapies have become commercially available, the monitoring of cerebral amyloid angiopathy (CAA), which is an important risk factor for amyloid-related imaging abnormalities, has received increasing attention. However, comprehensive evidence on the association between Alzheimer disease (AD) plasma biomarkers and various CAA imaging markers is still lacking.
Objective: To examine the association of CAA imaging markers with downstream AD plasma biomarkers in relation to amyloid-β (Aβ) uptake on positron emission tomography (PET) and whether their interplay is associated with cognitive changes.
Design, setting, and participants: This registry-based cohort study in 25 hospitals across South Korea recruited participants aged 45 years or older who were registered between January 1, 2016, and December 31, 2023. Participants were categorized as having no cognitive impairment, mild cognitive impairment, or dementia of the Alzheimer type.
Exposures: Cerebral amyloid angiopathy imaging markers assessed by magnetic resonance imaging, including cerebral microbleeds (CMBs), cortical superficial siderosis, white matter hyperintensities, lacunes, and enlarged perivascular spaces.
Main outcomes and measures: Plasma phosphorylated tau-217 (p-tau217) was measured using a commercial assay. Glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) were measured using a single-molecule assay on a single platform. All participants underwent amyloid PET imaging. Associations of CAA and vascular imaging markers with downstream AD plasma biomarkers were investigated using linear regression.
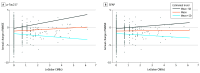
Results: A total of 1708 participants were included (mean [SD] age, 71.2 [8.4] years; 1044 female [61.1%]). The mean (SD) follow-up period was 4.3 (3.1) years. Lobar CMB counts and the presence of CAA were associated with downstream AD plasma biomarkers, including p-tau217 (β = 0.12 [95% CI, 0.05-0.18] and 0.29 [95% CI, 0.12-0.47], respectively), GFAP (β = 0.07 [95% CI, 0.03-0.12] and 0.20 [95% CI, 0.09-0.31], respectively), and NfL (β = 0.07 [95% CI, 0.03-0.11] and 0.16 [95% CI, 0.06-0.25], respectively) with and without the mediation of Aβ uptake on PET (indirect effect: lobar CMBs-p-tau217, 59.8% [β = 0.07 (95% CI, 0.03-0.11)]; lobar CMBs-GFAP, 49.3% [β = 0.04 (95% CI, 0.01-0.06)]; lobar CMBs-NfL, 20.9% [β = 0.01 (95% CI, 0.01-0.03)]; CAA-p-tau217, 50.9% [β = 0.15 (95% CI, 0.06-0.24)]; CAA-GFAP, 39.2% [β = 0.08 (95% CI, 0.03-0.13)]; CAA-NfL, 19.2% [β = 0.03 (95% CI, 0.01-0.05)]). Amyloid-β uptake fully mediated the associations between cortical superficial siderosis and downstream AD plasma markers. In contrast, hypertensive arteriosclerotic vascular imaging markers, including lacunes, deep CMBs, and enlarged perivascular spaces in basal ganglia, were associated with only NfL levels (β = 0.07 [95% CI, 0.01-0.13], 0.20 [95% CI, 0.08-0.32], and 0.14 [95% CI, 0.06-0.23], respectively), regardless of Aβ uptake on PET. Finally, there were interactive associations of lobar CMBs in conjunction with p-tau217 levels (β = -0.56 [95% CI, -0.79 to -0.34]) and GFAP levels (β = -0.44 [95% CI, -0.70 to -0.17]) with annual Mini-Mental State Examination changes.
Conclusions and relevance: In this cohort study of participants with no cognitive impairment, mild cognitive impairment, or dementia of the Alzheimer type, a novel association was found among CAA imaging markers, downstream AD plasma biomarkers, and cognitive declines in relation to brain Aβ burdens. The findings emphasize the importance of understanding the clinical effects of amyloid-related imaging abnormality-like CAA imaging markers in light of upcoming amyloid-targeted therapies.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

