Blocking C-terminal processing of KRAS4b via a direct covalent attack on the CaaX-box cysteine
- PMID: 40343987
- PMCID: PMC12088381
- DOI: 10.1073/pnas.2410766122
Blocking C-terminal processing of KRAS4b via a direct covalent attack on the CaaX-box cysteine
Abstract
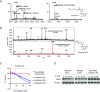
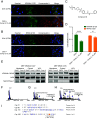
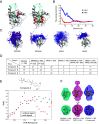
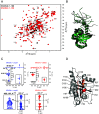
RAS is the most frequently mutated oncogene in cancer. RAS proteins show high sequence similarities in their G-domains but are significantly different in their C-terminal hypervariable regions (HVR). These regions interact with the cell membrane via lipid anchors that result from posttranslational modifications (PTM) of cysteine residues. KRAS4b is unique as it has only one cysteine that undergoes PTM, C185. Small molecule covalent modification of C185 would block any form of prenylation and subsequently inhibit attachment of KRAS4b to the cell membrane, blocking its biological activity. We translated this concept to the discovery and development of disulfide tethering screen hits into irreversible covalent modifiers of C185. These compounds inhibited proliferation of KRAS4b-driven mouse embryonic fibroblasts, but not cells driven by N-myristoylated KRAS4b that harbor a C185S mutation and are not dependent on C185 prenylation. Top-down proteomics was used to confirm target engagement in cells. These compounds bind in a pocket formed when the HVR folds back between helix 3 and 4 in the G-domain (HVR-α3-α4). This interaction can happen in the absence of small molecules as predicted by molecular dynamics simulations and is stabilized in the presence of C185 binders as confirmed by small-angle X-ray scattering and solution NMR. NOESY-HSQC, an NMR approach that measures internuclear distances of 6 Å or less, and structure analysis identified the critical residues and interactions that define the HVR-α3-α4 pocket. Further development of compounds that bind to this pocket could be the basis of a new approach to targeting KRAS cancers.
Keywords: C185; HVR; KRAS; KRAS4b; covalent inhibitor.
Conflict of interest statement
Competing interests statement:Reviewer M.R.P and co-author D.S. were co-authors of a review in 2023.
Figures
References
-
- Bos J. L., ras oncogenes in human cancer: A review. Cancer Res. 49, 4682–4689 (1989). - PubMed
-
- Downward J., Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3, 11–22 (2003). - PubMed
-
- Reuther G. W., Der C. J., The Ras branch of small GTPases: Ras family members don’t fall far from the tree. Curr. Opin. Cell Biol. 12, 157–165 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

