Structural maturation of the matrix lattice is not required for HIV-1 particle infectivity
- PMID: 40344051
- PMCID: PMC12063641
- DOI: 10.1126/sciadv.adv4356
Structural maturation of the matrix lattice is not required for HIV-1 particle infectivity
Abstract
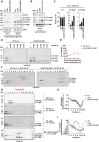
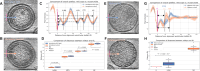
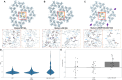
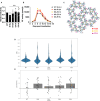
During HIV-1 maturation, the matrix (MA) lattice underlying the viral membrane undergoes a structural rearrangement, and the newly released capsid (CA) protein forms a mature CA. While it is well established that CA formation is essential for particle infectivity, the functional role of MA structural maturation remains unclear. Here, we examine maturation of an MA triple mutant, L20K/E73K/A82T, which, despite replicating similarly to wild-type (WT) in some cell lines, exhibits distinct biochemical behaviors that suggest altered MA-MA interactions. Cryo-electron tomography with subtomogram averaging reveals that, although the MA lattice in immature L20K/E73K/A82T virions closely resembles that of the WT, mature L20K/E73K/A82T virions lack a detectable MA lattice. All-atom molecular dynamics simulations suggest that this absence results from destabilized inter-trimer MA interactions in mature L20K/E73K/A82T mutant virions. These findings suggest that an ordered, membrane-associated mature MA lattice is not essential for HIV-1 infectivity, providing insights into the structural requirements for HIV-1 particle maturation and generation of infectious particles.
Figures



Update of
-
Structural maturation of the matrix lattice is not required for HIV-1 particle infectivity.bioRxiv [Preprint]. 2024 Dec 23:2024.12.22.629981. doi: 10.1101/2024.12.22.629981. bioRxiv. 2024. Update in: Sci Adv. 2025 May 9;11(19):eadv4356. doi: 10.1126/sciadv.adv4356. PMID: 39763880 Free PMC article. Updated. Preprint.
References
-
- C. Aiken, P. Zhang, in Advances in HIV-1 Assembly and Release (Springer, 2013), pp. 153–166.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

