A top-down insular cortex circuit crucial for non-nociceptive fear learning
- PMID: 40344067
- PMCID: PMC12063665
- DOI: 10.1126/sciadv.adt6996
A top-down insular cortex circuit crucial for non-nociceptive fear learning
Abstract
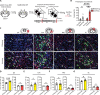
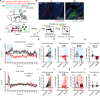
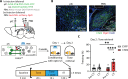
Understanding how threats drive fear memory formation is crucial to understanding how organisms adapt to environments and treat threat-related disorders such as PTSD. While traditional Pavlovian conditioning studies have provided valuable insights, the exclusive reliance on electric shock as a threat stimulus has limited our understanding of diverse threats. To address this, we developed a conditioning paradigm using a looming visual stimulus as an unconditioned stimulus (US) in mice and identified a distinct neural circuit for visual threat conditioning. Parabrachial CGRP neurons were necessary for both conditioning and memory retrieval. Upstream neurons in the posterior insular cortex (pIC) responded to looming stimuli, and their projections to the parabrachial nucleus (PBN) induced aversive states and drove conditioning. However, this pIC-to-PBN pathway was not required for foot-shock conditioning. These findings reveal how non-nociceptive visual stimuli can drive aversive states and fear memory formation, expanding our understanding of aversive US processing beyond traditional models.
Figures






References
-
- LeDoux J. E., Emotion, memory and the brain. Sci. Am. 270, 50–57 (1994). - PubMed
-
- Fanselow M. S., What is conditioned fear? Trends Neurosci. 7, 460–462 (1984).
-
- LeDoux J. E., Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000). - PubMed
-
- Medina J. F., Christopher Repa J., Mauk M. D., LeDoux J. E., Parallels between cerebellum-and amygdala-dependent conditioning. Nat. Rev. Neurosci. 3, 122–131 (2002). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

