Clinical Laboratory Experience With Prenatal cfDNA Screening in Triplet Pregnancies
- PMID: 40344231
- PMCID: PMC12137035
- DOI: 10.1002/pd.6812
Clinical Laboratory Experience With Prenatal cfDNA Screening in Triplet Pregnancies
Abstract
Objective: Prenatal cfDNA screening is the most sensitive and specific screen for common aneuploidies in singleton and twin pregnancies and has been endorsed by several professional societies as a first-tier screen or contingent screen. However, data for triplet pregnancies is lacking, as these pregnancies are relatively uncommon and obtaining sufficient data for a robust analysis of test performance is challenging.
Method: This study presents a retrospective review of over 1500 samples from triplet pregnancies screened via cfDNA for common aneuploidies.
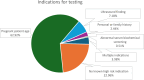
Results: Mean patient age was 34 years, while mean gestational age was 13 weeks. The most common indication for testing was patient age, representing > 60% of the cohort. There were 13 screen-positive cases (1.01%), 270 NR cases (17.32%), and the remainder were screen-negative. Complete or partial genetic and/or obstetric outcome information (including birth and neonatal outcomes) was available for 147 samples, including all 13 positive cfDNA samples. No false positive or false negative cases were identified.
Conclusion: The data from this study support the notion that cfDNA screening in triplet pregnancies is a reasonable approach given the lack of alternative screening options for these patients and that the performance likely approaches that of twin pregnancies, albeit with a higher no-call rate.
© 2025 The Author(s). Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors are current or former (SM) employees of Labcorp with the option to hold stock.
Figures
References
-
- American College of O, Gynecologists’ Committee on Practice B‐O, Committee on G, Society for Maternal‐Fetal MCommittee on GeneticsSociety for Maternal‐Fetal Medicine , “Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226,” Obstetrics & Gynecology 136, no. 4 (October 2020): e48–e69, 10.1097/AOG.0000000000004084. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous