Synergistic effects of the Aβ/fibrinogen complex on synaptotoxicity, neuroinflammation, and blood-brain barrier damage in Alzheimer's disease models
- PMID: 40344319
- PMCID: PMC12061846
- DOI: 10.1002/alz.70119
Synergistic effects of the Aβ/fibrinogen complex on synaptotoxicity, neuroinflammation, and blood-brain barrier damage in Alzheimer's disease models
Abstract
Introduction: Alzheimer's disease (AD) is characterized by amyloid-beta (Aβ), hyperphosphorylated tau, chronic neuroinflammation, blood-brain barrier (BBB) damage, and synaptic dysfunction, leading to neuronal loss and cognitive deficits. Vascular proteins, including fibrinogen, extravasate into the brain, further contributing to damage and inflammation. Fibrinogen's interaction with Aβ is well-established, but how this interaction contributes to synaptic dysfunction in AD is unknown.
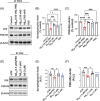
Methods: Organotypic hippocampal cultures (OHC) were exposed to Aβ42 oligomers, fibrinogen, or Aβ42/fibrinogen complexes. Synaptotoxicity was analyzed by Western blot. Aβ42 oligomers, fibrinogen, or their complexes were intracerebroventricularly injected into mice. Histopathological AD markers, synaptotoxicity, neuroinflammation, and vascular markers were observed by Western blot and immunofluorescence.
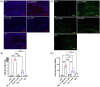
Results: Aβ42/fibrinogen complexes led to synaptic loss, tau181 phosphorylation, neuroinflammation, and BBB disruption, independent of Mac1/CD11b receptor signaling. Blocking Aβ42/fibrinogen complex formation prevented synaptotoxicity.
Discussion: These findings indicate that the Aβ42/fibrinogen complex has a synergistic impact on hippocampal synaptotoxicity and neuroinflammation.
Highlights: Fibrinogen binds to the central region of Aβ, forming a plasmin-resistant complex. The Aβ/fibrinogen complex induces synaptotoxicity, inflammation, and BBB disruption. Synaptotoxicity induced by the complex is independent of Mac1 receptor signaling.
Keywords: Alzheimer's disease; amyloid‐beta; blood‐brain barrier; fibrinogen; neuroinflammation; synaptotoxicity.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures



References
-
- Colom‐Cadena M, Davies C, Sirisi S, et al. Synaptic oligomeric tau in Alzheimer's disease—a potential culprit in the spread of tau pathology through the brain. Neuron. 2023;111(14):2170‐2183. e6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

