The Association of Neonatal Respiratory Distress With Ciliary Ultrastructure and Genotype in Primary Ciliary Dyskinesia
- PMID: 40344341
- PMCID: PMC12063519
- DOI: 10.1002/ppul.71091
The Association of Neonatal Respiratory Distress With Ciliary Ultrastructure and Genotype in Primary Ciliary Dyskinesia
Abstract
Objective: To evaluate the relationship between ciliary ultrastructure/genotype and prevalence of neonatal respiratory distress (NRD) in primary ciliary dyskinesia (PCD).
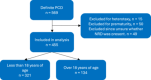
Study design: This was a retrospective analysis from a multicenter, prospective study of children and adults with PCD. Participants were classified by ultrastructural defect associated with their diagnostic genetic variants: 1) outer dynein arm defect alone (ODA), 2) outer plus inner dynein arm defect (ODA/IDA), 3) inner dynein arm defect with microtubular disorganization (IDA/MTD), 4) DNAH11 (encodes ODA protein but has normal ultrastructure), and 5) normal/near-normal/other. The likelihood of NRD between ultrastructure groups or genotypes was evaluated by multivariate analysis using logistic regression, controlled for age, gender, race, and variant type. Similar analysis was performed within individual genotypes to assess association of NRD with the presence of 2 loss-of-function variants.
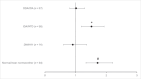
Results: Of the 455 participants analyzed, 305 (67.0%) reported NRD. The odds ratio for NRD in the DNAH11 group was significantly lower (OR: 0.35, 95% CI: 0.16-0.76) compared to NRD in the ODA group. Within the DNAH5 group, those with two loss-of-function variants were more likely to have NRD compared to those with possible residual function variants (OR: 3.06, 95% CI: 1.33-7).
Conclusion: NRD is less common in those with DNAH11 variants, thus a high index of suspicion should remain for PCD in the absence of NRD. Variant type (loss-of-function vs. residual function) may explain phenotypic variability within individual PCD genes.
Keywords: genotype/phenotype correlation; neonatal respiratory distress; primary ciliary dyskinesia.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
Stephanie D. Davis has grant support from the NIH and ReCode Therapeutics as well as support from the Primary Ciliary Dyskinesia Foundation. She also serves as a member of the of the Primary Ciliary Dyskinesia Medical and Scientific Advisory Council. Thomas W. Ferkol has grant support from the NIH (HL096458, TR003860, TR3860, AI146999, HL125241, U01HL172658, HG009650) as well as support from ReCode Therapeutics and Parion Sciences and also serves as a consultant for TransBio and Arrowhead Pharmaceuticals. He serves on the Clinical Study Advisory Board for ReCode Therapeutics. Adam J. Shapiro has received support from the Chest Foundation, the Primary Ciliary Dyskinesia Foundation, and serves as a consultant for Parion Sciences and Ethris GMBH. He serves as the medical director of the Primary Ciliary Dyskinesia Foundation and also has participated on a board for ReCode Therapeutics. Kenneth Olivier has received research grants from ReCode Therapeutics and serves on the Medical and Scientific Advisory Council of the Primary Ciliary Dyskinesia Foundation. Margaret Rosenfeld receives grant support from the NIH (U54 HL096458). Scott D. Sagel has grant support from the NIH (U54 HL096458, UL1 TR002535, R21 TR004057) and serves as a consultant for Pharming Healthcare/Precision Medicine Group. Sharon D. Dell has grant support from the BCCHRI Establishment Award and the NIH (U54 HL096458). She serves as a consultant for Sanofi and Regeneron Pharmaceuticals. She has received honoraria for speaking to AstraZeneca Canada, Sanofi Aventis Canada, and Sanofi Genzyme Corporation. She serves on the scientific advisory board of Sanofi Aventis Canada and as Deputy Editor of Annals ATS. She receives support for clinical trials from Boehringer Ingelheim Canada, Sanofi Aventis Canada, Vertex Pharmaceuticals, Merck Research Laboratories, and GlaxoSmithKline UK. Maimoona A. Zariwala receives grant support from the NIH (U54 HL096458, R01 HL071798). She serves on the Primary Ciliary Dyskinesia Medical and Scientific Advisory Council. Margaret W. Leigh has received grant support from the Primary Ciliary Dyskinesia Foundation and serves on the board of directors for the Primary Ciliary Dyskinesia Foundation.
Figures
References
-
- Noone P. G., Leigh M. W., Sannuti A., et al., “Primary Ciliary Dyskinesia: Diagnostic and Phenotypic Features,” American Journal of Respiratory and Critical Care Medicine 169, no. 4 (February 2004): 459–467. - PubMed
-
- Ferkol T. and Leigh M., “Primary Ciliary Dyskinesia and Newborn Respiratory Distress,” in Seminars in Perinatology. (WB Saunders, December 2006), 335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- U54 HL096458/HL/NHLBI NIH HHS/United States
- Supported by the National Institute of Health (NIH) U54HL096458 funded by the Office of Rare Disease Research (National Center for Advancing Translational Science) and administered by NHLBI and the Carolina For The Kids Grant Program, US NIH/NHLBI grant R01HL071798 to MRK and MAZ, NIH/NCATS Colorado CTSA Grant Number UM1 TR004399. This study was performed at the University of North Carolina at Chapel Hill under IRB numbers 05-2979 and 14-1225.
- R01 HL071798/HL/NHLBI NIH HHS/United States
- UM1 TR004399/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources

