Immune and Angiogenic Profiling of Mesenchymal Stem Cell Functions in a Subcutaneous Microenvironment for Allogeneic Islet Transplantation
- PMID: 40344470
- PMCID: PMC12120776
- DOI: 10.1002/advs.202411574
Immune and Angiogenic Profiling of Mesenchymal Stem Cell Functions in a Subcutaneous Microenvironment for Allogeneic Islet Transplantation
Abstract
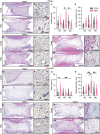
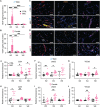
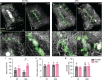
Islet transplantation offers a promising treatment for type 1 diabetes (T1D), by aiming to restore insulin production and improve glycemic control. However, T1D is compounded by impaired angiogenesis and immune dysregulation, which hinder the therapeutic potential of cell replacement strategies. To address this, this work evaluates the proangiogenic and immunomodulatory properties of mesenchymal stem cells (MSCs) to enhance vascularization and modulate early-stage immune rejection pathways in the context of islet allotransplantation. This work employs the Neovascularized Implantable Cell Homing and Encapsulation (NICHE) platform, a subcutaneous vascularized implant with localized immunomodulation developed by the group. This study assesses vascularization and immune regulation provided by MSCs, aiming to improve islet survival and integration in diabetic rats while considering sex as a biological variable. These findings demonstrate that MSCs significantly enhance vascularization and modulate the local microenvironment during the peri-transplant period. Importantly, this work discovers sex-specific differences in both processes, which influence islet engraftment and long-term function.
Keywords: Type 1 diabetes; immunomodulation; islet engraftment; mesenchymal stem cells; sex‐specific differences; vascularized subcutaneous microenvironment.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
S.C., M.F., C.Y.X.C., and A.G. are inventors of intellectual property licensed by Continuity Biosciences. AG is a co‐founder and scientific advisor of Continuity Biosciences. The other authors declare no conflict of interest.
Figures





References
-
- a) Nichols J. E., La Francesca S., Niles J. A., Vega S. P., Argueta L. B., Frank L., Christiani D. C., Pyles R. B., Himes B. E., Zhang R., Li S., Sakamoto J., Rhudy J., Hendricks G., Begarani F., Liu X., Patrikeev I., Pal R., Usheva E., Vargas G., Miller A., Woodson L., Wacher A., Grimaldo M., Weaver D., Mlcak R., Cortiella J., Sci. Transl. Med. 2018, 10, aao3926; - PubMed
- b) Abreu S. C., Weiss D. J., Rocco P. R., Stem Cell Res. Ther. 2016, 7, 53; - PMC - PubMed
- c) Ionescu L., Byrne R. N., van Haaften T., Vadivel A., Alphonse R. S., Rey‐Parra G. J., Weissmann G., Hall A., Eaton F., Thebaud B., Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L967; - PMC - PubMed
- d) Lee J. W., Fang X., Krasnodembskaya A., Howard J. P., Matthay M. A., Stem Cells 2011, 29, 913; - PMC - PubMed
- e) Tao H., Han Z., Han Z. C., Li Z., Stem Cells Int. 2016, 2016, 1314709; - PMC - PubMed
- f) Thakker R., Yang P., Curr. Treat. Options Cardiovasc. Med. 2014, 16, 323; - PMC - PubMed
- g) Watt S. M., Gullo F., van der Garde M., Markeson D., Camicia R., Khoo C. P., Zwaginga J. J., Br. Med. Bull. 2013, 108, 25. - PMC - PubMed
-
- a) Grattoni A., Korbutt G., Tomei A. A., Garcia A. J., Pepper A. R., Stabler C., Brehm M., Papas K., Citro A., Shirwan H., Millman J. R., Melero‐Martin J., Graham M., Sefton M., Ma M., Kenyon N., Veiseh O., Desai T. A., Nostro M. C., Marinac M., Sykes M., Russ H. A., Odorico J., Tang Q., Ricordi C., Latres E., Mamrak N. E., Giraldo J., Poznansky M. C., de Vos P., Nat. Rev. Endocrinol. 2025, 21, 14; - PMC - PubMed
- b) Yeung T. Y., Seeberger K. L., Kin T., Adesida A., Jomha N., Shapiro A. M., Korbutt G. S., PLoS One 2012, 7, 38189; - PMC - PubMed
- c) Berman D. M., Willman M. A., Han D., Kleiner G., Kenyon N. M., Cabrera O., Karl J. A., Wiseman R. W., O'Connor D. H., Bartholomew A. M., Kenyon N. S., Diabetes 2010, 59, 2558; - PMC - PubMed
- d) Ding Y., Xu D., Feng G., Bushell A., Muschel R. J., Wood K. J., Diabetes 2009, 58, 1797; - PMC - PubMed
- e) Ben Nasr M., Vergani A., Avruch J., Liu L., Kefaloyianni E., D'Addio F., Tezza S., Corradi D., Bassi R., Valderrama‐Vasquez A., Usuelli V., Kim J., Azzi J., El Essawy B., Markmann J., Abdi R., Fiorina P., Acta Diabetol. 2015, 52, 917; - PMC - PubMed
- f) Razavi M., Wang J., Thakor A. S., Sci. Adv. 2021, 7, abf9221; - PMC - PubMed
- g) Vaithilingam V., Evans M. D. M., Lewy D. M., Bean P. A., Bal S., Tuch B. E., Sci. Rep. 2017, 7, 10059. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
