Strong and Fatigue-Resistant Hydrogels via Poor Solvent Evaporation Assisted Hot-Stretching for Tendon Repair
- PMID: 40344510
- PMCID: PMC12302550
- DOI: 10.1002/advs.202503697
Strong and Fatigue-Resistant Hydrogels via Poor Solvent Evaporation Assisted Hot-Stretching for Tendon Repair
Abstract
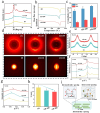
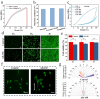
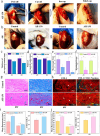
It is highly desirable but still remains challenging to develop high-performance hydrogels with satisfactory mechanical properties for tissue engineering. Here, anisotropic yet transparent hydrogels (AHs) are prepared for tendon repair via a facile "poor solvent evaporation assisted hot-stretching" strategy. AHs have great mechanical properties with tensile strength, toughness, and fracture energy as high as 33.14 ± 2.05 MPa, 44.1 ± 3.5 MJ m-3, and 106.18 ± 7.2 kJ m-2, respectively. Especially, AHs show unique flaw-insensitive characteristics, and cracks can only deflect along the fiber alignment direction rather than propagate transverse to this direction, showing an interesting self-protection function. The high strength, toughness, and fatigue resistance originate from the hierarchal structure of AHs, i.e., the densified polymeric network comprising fiber bundles and nanofibrils with aligned macromolecular chains, crystalline domains, and intermolecular hydrogen bonds. AHs with superior biocompatibility and swelling resistance can be used to repair rat tendons, and implantation of AHs can promote collagen regeneration for the tendon repair. This study provides a new method to fabricate strong and anti-fatigue hydrogels as a new class of promising materials for soft tissues.
Keywords: anisotropic hydrogels; flaw‐insensitivity; hot‐stretching; poor solvent evaporation; tendon repair.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang Z., Xu X., Tan R., Zhang S., Zhang K., Hu J., Adv. Funct. Mater. 2024, 34, 2312667.
-
- Han S., Wu Q., Zhu J., Zhang J., Chen A., Su S., Liu J., Huang J., Yang X., Guan L., Mater. Horiz. 2023, 10, 1012. - PubMed
-
- Wang Z., Zhang Y., Yin Y., Liu J., Li P., Zhao Y., Bai D., Zhao H., Han X., Chen Q., Adv. Mater. 2022, 34, 2108300. - PubMed
-
- Wu Y., Zhang Y., Wu H., Wen J., Zhang S., Xing W., Zhang H., Xue H., Gao J., Mai Y., Adv. Mater. 2023, 35, 2210624. - PubMed
MeSH terms
Substances
Grants and funding
- 52473049/National Natural Science Foundation of China
- 2022YFB3808000/National Key R&D Program of China
- Jiangsu Provincial International Joint Laboratory of Optic/Electronic/Magnetic Functional Materials and Sensors
- Qing Lan Project of Yangzhou University and Jiangsu Province
- BK20240934/Natural Science Foundation of Jiangsu Province
LinkOut - more resources
Full Text Sources
