An Intelligent and Conductive Hydrogel with Multiresponsive and ROS Scavenging Properties for Infection Prevention and Anti-Inflammatory Treatment Assisted by Electrical Stimulation for Diabetic Wound
- PMID: 40344517
- PMCID: PMC12199324
- DOI: 10.1002/advs.202500696
An Intelligent and Conductive Hydrogel with Multiresponsive and ROS Scavenging Properties for Infection Prevention and Anti-Inflammatory Treatment Assisted by Electrical Stimulation for Diabetic Wound
Abstract
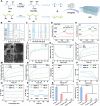
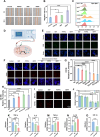
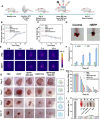
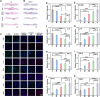
Diabetic wounds experience a hyperglycemic, hypoxic environment, combined with ongoing oxidative stress and inflammatory imbalances, significantly disrupts normal healing process. Advanced hydrogels have been considered one of the most exciting medical biomaterials for the potential in wounds healing. Herein, a novel conductive hydrogel (HEPP), designed to release nanozyme (PTPPG) in response to its microenvironment, was created to facilitate glucose (Glu) catabolism. Furthermore, the HEPP integrates photodynamic therapy (PDT), photothermal therapy (PTT), and self-cascading reactive oxygen species (ROS) to prevent bacterial infections while ensuring a continuous supply of oxygen (O2) to the wound. The HEPP not only adeptly controls high ROS levels, but also enhances the regulation of inflammation in the wound area via electrical stimulation (ES), thereby promoting healing that is supported by the immune response. Studies conducted in vitro, along with transcriptomic analyses, indicate that ES primarily mitigates inflammation by regulating Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). The effects of HEPP combined with ES are primarily connected to their impact on TNF signaling pathways. By reducing the formation of ROS and employing ES to effectively lessen inflammation, this approach offers an innovative method to manage complicated diabetic wounds, ulcers, and a range of inflammatory conditions linked to infections.
Keywords: ROS scavenging; antibacterial; conductive hydrogel; diabetic wound; electrical stimulation; multiple responses; synergistic anti‐inflammatory.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
