MiR-129-5p alleviates depression and anxiety by increasing astrocyte ATP production partly through targeting deubiquitinase Mysm1
- PMID: 40344568
- PMCID: PMC12064192
- DOI: 10.1371/journal.pone.0322715
MiR-129-5p alleviates depression and anxiety by increasing astrocyte ATP production partly through targeting deubiquitinase Mysm1
Abstract
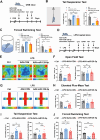
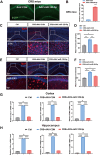
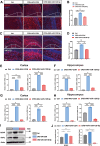
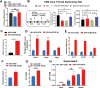
Major depressive disorder (MDD) is a major global mental concern that severely affects quality of life, yet current pharmacological treatments remain limited in their effectiveness. Long-term chronic stress has been shown to increase the incidence of depression and anxiety. Micro RNAs (miRNAs) have been revealed to participate in the pathological process of depression and represent promising therapeutic targets. In this study, we found that microRNA-129-5p (miR-129-5p) was significantly decreased in the brains of depressive mice. Overexpression of miR-129-5p in the hippocampus effectively alleviated depressive-like behaviors and reduced the activation of microglial cells and astrocytes. In addition, ATP levels in depressive mice were significantly increased following miR-129-5p overexpression. The antidepressant effects of miR-129-5p were reversed when ATP function was blocked with the non-specific P2 receptor antagonist suramin. In vitro experiments revealed that miR-129-5p overexpression enhanced ATP production in astrocytes. Furthermore, using a dual-luciferase reporter assay, we found that miR-129-5p directly targeted Mysm1. When overexpressed in astrocytes, miR-129-5p significantly suppressed Mysm1 expression, promoted phosphorylation of p53 and AMPK, and enhanced the expression of PGC1α, factors previously associated with ATP production. Our findings highlight the crucial role of miR-129-5p in regulating depression, suggesting that miR-129-5p overexpression may serve as an effective strategy for antidepressant treatment.
Copyright: © 2025 Qin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Thapar A, Eyre O, Patel V, et al. Depression in young people. Lancet. 2022;400(10352):617-31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

