Neuronal FGF13 Inhibits Mitochondria-Derived Damage Signals to Prevent Neuroinflammation and Neurodegeneration in a Mouse Model of Parkinson's Disease
- PMID: 40344619
- PMCID: PMC12302648
- DOI: 10.1002/advs.202503579
Neuronal FGF13 Inhibits Mitochondria-Derived Damage Signals to Prevent Neuroinflammation and Neurodegeneration in a Mouse Model of Parkinson's Disease
Abstract
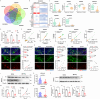
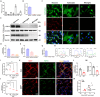
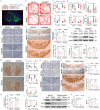
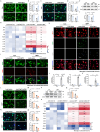
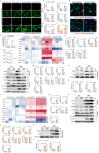
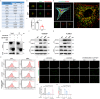
Fibroblast growth factor homologous factors (FHFs) are highly expressed in the central nervous system (CNS). It is demonstrated that the FHFs subfamily plays cardinal roles in several neuropathological diseases, while their involvement in Parkinson's disease (PD) has been so far scarcely investigated. From the publicly available Gene Expression Omnibus (GEO) datasets, FHF2 (also known as fibroblast growth factor 13, FGF13) alterations are described in PD patients. Fgf13 gene is significantly decreased in several PD mouse models, and its overexpression alleviates the PD-like pathological phenotype. Although FGF13 is highly expressed in neurons, it functions by preventing glia-dependent inflammatory processes. Mechanistically, FGF13 combines mitochondrial proteins such as MCHT2 (a protein localized on the mitochondrial outer membrane), to anchor mitochondria within the cytoplasm. Under PD-related stress, decreased neuronal FGF13 levels induce the release of the damaged mitochondria, which in turn activate microglia and astrocytes, thereby promoting neurodegeneration. Abacavir, an FDA-applied anti-retroviral drug, is identified to prevent excessive gliosis and neuron loss in both glia-neuron co-cultures and PD mouse models by rejuvenating FGF13 signaling. Collectively, neuronal FGF13 inhibits the transfer of stressed mitochondria to glia, thereby impeding neuroinflammation and neurodegeneration. Abacavir is a promising neuroprotectant and sets a brake to PD progression.
Keywords: FGF13; MTCH2; mitochondrial transfer; neuroinflammation; parkinson's disease.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
