Attenuated Nuclear Tension Regulates Progerin-Induced Mechanosensitive Nuclear Wrinkling and Chromatin Remodeling
- PMID: 40344643
- PMCID: PMC12376529
- DOI: 10.1002/advs.202502375
Attenuated Nuclear Tension Regulates Progerin-Induced Mechanosensitive Nuclear Wrinkling and Chromatin Remodeling
Abstract
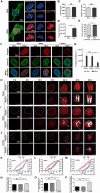
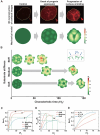
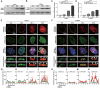
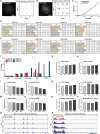
Hutchinson-Gilford progeria syndrome, caused by a mutation in the LMNA gene, leads to increased levels of truncated prelamin A, progerin, in the nuclear membrane. The accumulation of progerin results in defective nuclear morphology and is associated with altered expression of linker of the nucleoskeleton and cytoskeleton complex proteins, which are critical for nuclear signal transduction via molecular coupling between the extranuclear cytoskeleton and lamin-associated nuclear envelope. However, the molecular mechanisms underlying progerin accumulation-induced nuclear deformation and its effects on intranuclear chromosomal organization remain unclear. Here, the spatiotemporal evolution of nuclear wrinkles is analyzed in response to variations in substrate stiffness using a doxycycline-inducible progerin expression system. It is found that cytoskeletal tension regulates the onset of progerin-induced nuclear envelope wrinkling and that the molecular interaction between SUN1 and LMNA controls the actomyosin-dependent attenuation of nuclear tension. Genome-wide analysis of chromatin accessibility and gene expression further suggests that an imbalance in force between the intra- and extranuclear spaces induces nuclear deformation, which specifically regulates progeria-associated gene expression via modification of mechanosensitive signaling pathways. The findings highlight the crucial role of nuclear lamin-cytoskeletal connectivity in bridging nuclear mechanotransduction and the biological aging process.
Keywords: Hutchinson–Gilford progeria syndrome; LINC complex; SUN1; actomyosin contractility; chromatin remodeling; heterochromatin; mechanosensation; nuclear deformation; nuclear tension; nuclear wrinkling; progerin.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Barrowman J., Wiley P. A., Hudon‐Miller S. E., Hrycyna C. A., Michaelis S., Hum. Mol. Genet. 2012, 21, 4084; - PMC - PubMed
- b) Fong L. G., Ng J. K., Meta M., Coté N., Yang S. H., Stewart C. L., Sullivan T., Burghardt A., Majumdar S., Reue K., Proc. Natl. Acad. Sci. USA 2004, 101, 18111. - PMC - PubMed
-
- a) Goldman R. D., Shumaker D. K., Erdos M. R., Eriksson M., Goldman A. E., Gordon L. B., Gruenbaum Y., Khuon S., Mendez M., Varga R., Proc. Natl. Acad. Sci. USA 2004, 101, 8963; - PMC - PubMed
- b) Dahl K. N., Scaffidi P., Islam M. F., Yodh A. G., Wilson K. L., Misteli T., Proc. Natl. Acad. Sci. USA 2006, 103, 10271. - PMC - PubMed
-
- Chen Z.‐J., Wang W.‐P., Chen Y.‐C., Wang J.‐Y., Lin W.‐H., Tai L.‐A., Liou G.‐G., Yang C.‐S., Chi Y.‐H., J. Cell Sci. 2014, 127, 1792. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
