Effect of Early Treatment of Spasticity After Stroke on Motor Recovery: Protocol for the Baclotox Multicenter, Double-Blind, Double-Dummy Randomized Controlled Trial
- PMID: 40344664
- PMCID: PMC12102626
- DOI: 10.2196/62951
Effect of Early Treatment of Spasticity After Stroke on Motor Recovery: Protocol for the Baclotox Multicenter, Double-Blind, Double-Dummy Randomized Controlled Trial
Abstract
Background: Individuals with poststroke hemiplegia often develop spasticity, which increases disability. Antispastic treatments such as baclofen and botulinum toxin are commonly prescribed in poststroke recovery. However, their impact on motor recovery, especially when administered within the first 2 months after stroke, remains unclear.
Objective: This study aims to compare the motor recovery effects of botulinum toxin versus oral baclofen. The hypothesis is that botulinum toxin is more supportive of motor recovery than baclofen and enhances functional recovery.
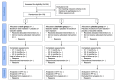
Methods: The study is a multicenter, controlled phase IV, comparative, prospective, randomized, double-blind, double-dummy, superiority trial to compare the toxin and baclofen, and a noninferiority trial to compare the toxin and the placebo. It focuses on the time course of the Fugl-Meyer Motor Assessment (FMA) as the primary outcome. The main inclusion criterion is patients with a single stroke in the past 2 months. Treatment comprises 1 intramuscular injection at treatment initiation and oral tablets for 4 months. Randomized patients are allocated to 3 arms: botulinum toxin with placebo baclofen, baclofen with placebo botulinum toxin, and placebo baclofen with placebo botulinum toxin. FMA scores are assessed at pretreatment, 1 month, and 3 months later. Spasticity, functional abilities, activities of daily living, pain, and quality of life are also evaluated. Adverse effects are monitored. A positive difference of 13 points in the FMA time course between the botulinum toxin and baclofen groups is considered a relevant effect. The data analysis plan involves linear regression models to compare primary and secondary outcomes, with adjustments for covariates such as age, center, and associated treatments. Subgroup analyses will examine proportional recovery profiles, and missing data in Fugl-Meyer scores will be addressed using imputation methods.
Results: A total of 179 participants were randomized across 18 centers, with inclusions delayed due to the COVID-19 pandemic. As of December 2024, the data manager currently has all the data, and a review of data quality is in progress. No statistical analysis has been conducted so far, and the blind will be lifted after the analysis.
Conclusions: This study identifies the most suitable spasticity treatment, considering the specificities of the stroke and constraints during the recovery phase. It will provide recommendations for the primary treatment of early spasticity post stroke.
Trial registration: ClinicalTrials.gov NCT02462317; https://clinicaltrials.gov/study/NCT02462317; European clinical trials (EudraCT) 2010-022881-28; https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-022881-28/FR.
International registered report identifier (irrid): DERR1-10.2196/62951.
Keywords: baclofen; botulinum toxin A; hemiplegia; motor recovery; muscle spasticity; rehabilitation; stroke.
©Emmeline Montane, Nabila Brihmat, Camille Cormier, Claire Thalamas, Vanessa Rousseau, Gerard Tap, Xavier De Boissezon, Evelyne Castel-Lacanal, Baclotox Group, Philippe Marque. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 09.05.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CMM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi: 10.1016/s0140-6736(13)61953-4. https://europepmc.org/abstract/MED/24449944 S0140-6736(13)61953-4 - DOI - PMC - PubMed
-
- Lyrer PA. [Epidemiology of stroke] Bull Med Suisses. 2000;81(37):2082–2085. doi: 10.4414/bms.2000.07606. - DOI
-
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST, Corbett D, Cramer SC. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12(5):444–450. doi: 10.1177/1747493017711816. https://journals.sagepub.com/doi/abs/10.1177/1747493017711816?url_ver=Z3... - DOI - DOI - PubMed
-
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. https://linkinghub.elsevier.com/retrieve/pii/S0960-9822(11)00125-4 S0960-9822(11)00125-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

