Real-World Efficacy Profile of Compassionate Use of Asciminib in an Italian, Multi-Resistant Chronic-Phase Chronic Myeloid Leukemia (CML-CP) Patient Population
- PMID: 40344674
- PMCID: PMC12064212
- DOI: 10.1002/hon.70101
Real-World Efficacy Profile of Compassionate Use of Asciminib in an Italian, Multi-Resistant Chronic-Phase Chronic Myeloid Leukemia (CML-CP) Patient Population
Abstract
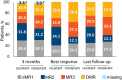
Chronic myeloid leukemia (CML) patients who have experienced failure and/or intolerance to multiple lines of treatment have limited therapeutic possibilities. Asciminib is a first-in-class tyrosine kinase inhibitor (TKI) that inhibits the ABL Myristoyl Pocket (STAMP or Specifically Targeting the ABL Myristoyl Pocket) within the BCR::ABL1 oncoprotein. This retrospective Italian analysis reports the efficacy and safety outcomes of asciminib in treating 77 CML patients in chronic phase (CML-CP) within a compassionate use setting. Patients were heavily pretreated with a median of 3 TKIs (55.8% had prior ponatinib exposure). Overall, 57.1% and 42.9% patients switched to asciminib because of resistance and intolerance, respectively. Asciminib maintained or improved molecular responses (MRs) in most patients: as best response, 41 patients (53%) achieved a MR3 or better, with 25 patients (32.5%) reaching deep molecular response (DMR). Greater percentages of intolerant patients achieved MR compared with resistant patients, although the probability of reaching at least a MR3 was not significant between the two groups (p = 0.116). Patients with the T315I mutation responded to asciminib, while ponatinib pre-treated patients showed lower MR improvements compared to naïve patients and had a lower probability to reach a MR3 versus naïve patients (p = 0.0262). These results highlight asciminib remarkable tolerability and efficacy in real-world CML-CP patient population, including heavily pretreated patients, those intolerant and resistant to previous TKIs, and presenting several comorbidities. TRAIL REGISTRATION: The identification code for the MAP is CABL001AIT01M.
Keywords: TKI resistance/intolerance; asciminib; chronic myeloid leukemia in chronic phase (CML‐CP); major molecular response (MMR); real‐world; tyrosine kinase inhibitor (TKI).
© 2025 The Author(s). Hematological Oncology published by John Wiley & Sons Ltd.
Conflict of interest statement
A.P.N., A.M., P.C. are Novartis employees.
Figures



References
-
- Akard L., Albitar M., Hill C. E., and Pinilla‐Ibarz J., “The ‘Hit Hard and Hit Early’ Approach to the Treatment of Chronic Myeloid Leukemia: Implications of the Updated National Comprehensive Cancer Network Clinical Practice Guidelines for Routine Practice,” Clinical Advances in Hematology and Oncology 11 (2013): 421–432.
-
- Jabbour E. J., Cortes J. E., and Kantarjian H. M., “Resistance to Tyrosine Kinase Inhibition Therapy for Chronic Myelogenous Leukemia: A Clinical Perspective and Emerging Treatment Options,” Clinical Lymphoma, Myeloma & Leukemia 13, no. 5 (2013): 515–529, 10.1016/j.clml.2013.03.018. - DOI - PMC - PubMed
-
- Breccia M., Olimpieri P. P., Olimpieri O., et al., “How Many Chronic Myeloid Leukemia Patients Who Started a Frontline Second‐Generation Tyrosine Kinase Inhibitor Have to Switch to a Second‐Line Treatment? A Retrospective Analysis From the Monitoring Registries of the Italian Medicines Agency (AIFA),” Cancer Medicine 9, no. 12 (2020): 4160–4165, 10.1002/cam4.3071. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

