Blood-brain barrier injury and neuroinflammation in pre-eclampsia and eclampsia
- PMID: 40344719
- PMCID: PMC12136835
- DOI: 10.1016/j.ebiom.2025.105742
Blood-brain barrier injury and neuroinflammation in pre-eclampsia and eclampsia
Abstract
Background: Cerebral complications of pre-eclampsia are a leading cause of maternal mortality. Better understanding of the pathophysiology may enable the development of novel strategies to protect the maternal brain. We aimed to investigate blood-brain barrier injury and neuroinflammatory pathways in women with eclampsia and pre-eclampsia compared to normotensive pregnancies.
Methods: This observational cross-sectional study conducted between March 2021 and June 2023, included women with eclampsia, pre-eclampsia, and normotensive pregnancies admitted to Tygerberg Hospital, Cape Town, South Africa who underwent caesarean delivery. Cerebrospinal fluid and plasma samples were collected during caesarean delivery. Blood-brain barrier injury was assessed using immunonephelometry for albumin and ELISA assays for claudin-5 and matrix metalloproteinase-9 (MMP-9). Neuroinflammatory markers were analysed on the multiplex Bio-Plex Pro Human Cytokine-Screening assay. Data were analysed using parametric methods after log transformation and are presented as fold changes (geometric mean ratios) between groups.
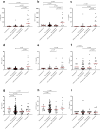
Findings: The study included 129 women: Eleven had eclampsia, 17 had pre-eclampsia with end-organ complications, 88 had pre-eclampsia without end-organ complications, and 13 with normotensive pregnancies. Women with eclampsia had increased cerebrospinal fluid concentrations of claudin-5 (2.7-fold, 95% CI 1.4-5.1, p = 0.002 vs normotensive control) and MMP-9 (2.5-fold, 95% CI 1.1-5.3, p = 0.024 vs pre-eclampsia with end-organ complications). They also demonstrated increased cerebrospinal fluid cytokine levels compared to normotensive controls, reflecting inflammatory recruitment (Interleukin-8: 7.2-fold, 95% CI 2.7-18.5, p < 0.001), cytotoxicity (Interleukin-6: 20.7-fold, 95% CI 6.4-63.6, p < 0.001), and immune modulation (Interleukin-10: 2.0-fold, 95% CI 1.2-3.1, p = 0.004). Neuroprotective markers were reduced in eclampsia (stem cell factor: 0.5-fold, 95% CI 0.3-0.8, p = 0.005) compared to normotensive controls. There was no correlation between cytokine concentrations in the cerebrospinal fluid and plasma. Women with pre-eclampsia showed less pronounced changes indicative of blood-brain barrier injury and immune modulation.
Interpretation: Eclampsia is associated with blood-brain barrier injury and acute neuroinflammation originating from cerebral tissue, inducing cytotoxicity. This may be an underlying mechanism for seizures and cerebral injury in eclampsia and pre-eclampsia.
Funding: This study was supported by the Swedish Research Council, Herbert och Karin Jacobsson Stiftelse, Wilhelm and Martina Lundgren Foundations, and Swedish Society Of Medicine.
Keywords: Blood-brain barrier; Eclampsia; Maternal complications; Neuroinflammation; Pre-eclampsia.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Enigma, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is together with KB a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). HZ is chair of the Alzheimer's Association Global Biomarker Standardisation Consortium and chair of the IFCC WG-BND. HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023-00356; #2022-01018; and #2019-02397), the European Union's Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-128438-C), the Bluefield Project, Cure Alzheimer's Fund, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen fӧr Gamla Tjӓnarinnor, Hjӓrnfonden, Sweden (#FO2022-0270), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, the UK Dementia Research Institute at UCL (UKDRI-1003), and an anonymous donor. KB has served at scientific advisory boards and/or as a consultant for Abbvie, AC Immune, ALZpath, Aribio, Beckman Coulter, BioArctic, Biogen, Eisai, Lilly, Neurimmune, Ono Pharma, Prothena, Roche Diagnostics, Sanofi, Siemens Healthineers, Novartis, and Julius Clinical. KB has received payments from Biogen, Eisai, and Roche Diagnostics for participation in educational programs. KB has received grants paid to the institute from Swedish Research Council (#2017-00915 and #2022-00732), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-965240 and #ALFGBG-1006418), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, #AF-968270, and #AF-994551), Hjärnfonden, Sweden (#ALZ2022-0006, #FO2024-0048-TK-130, and FO2024-0048-HK-24), the Alzheimer's Association 2021 Zenith Award (ZEN-21-848495), the Alzheimer's Association 2022-2025 Special Grant (SG-23-1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, Familjen Rӧnstrӧms Stiftelse, Stockholm, Sweden To the Institute. LB has collaborated with Roche, PerkinElmer, and Thermo Fischer as sponsors for PlGF and sFlt-1 reagents (IMPACT study). LB has obtained reimbursement for lecture by iLab Medical and Thermo Fisher, expert opinion from Homburg and Partner. LB has received trial drug and placebo from Merck.
Figures

References
-
- Chappell L.C., Cluver C.A., Kingdom J., Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–354. - PubMed
-
- Say L., Chou D., Gemmill A., et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. - PubMed
-
- Fishel Bartal M., Sibai B.M. Eclampsia in the 21st century. Am J Obstet Gynecol. 2022;226(2S):S1237–S1253. - PubMed
-
- Zeeman G.G. Neurologic complications of pre-eclampsia. Semin Perinatol. 2009;33(3):166–172. - PubMed
-
- Brohan M.P., Daly F.P., Kelly L., et al. Hypertensive disorders of pregnancy and long-term risk of maternal stroke—a systematic review and meta-analysis. Am J Obstet Gynecol. 2023;229(3):248–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

