Early switch from run-in with targeted to immunotherapy in advanced BRAFV600-positive melanoma: final results of the randomised phase II ImmunoCobiVem trial
- PMID: 40345056
- PMCID: PMC12136786
- DOI: 10.1016/j.esmoop.2025.105053
Early switch from run-in with targeted to immunotherapy in advanced BRAFV600-positive melanoma: final results of the randomised phase II ImmunoCobiVem trial
Abstract
Background: Optimal sequencing of immune checkpoint inhibitors (ICIs) and targeted therapies (TTs) in BRAFV600-positive advanced melanoma should achieve rapid tumour control and durable progression-free survival (PFS), translating into prolonged overall survival (OS).
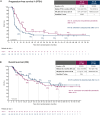
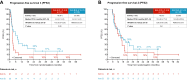
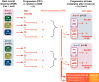
Patients and methods: The 1 : 1 randomised phase II ImmunoCobiVem trial compared-after a 3-month run-in phase with vemurafenib (VEM, 960 mg twice daily) and cobimetinib (COB, 60 mg daily days 21-28, q4w)-continuous VEM + COB until disease progression (PD1) and second-line atezolizumab (ATEZO, 1200 mg, q3w) in arm A versus early switch to ATEZO after run-in, followed by crossover to VEM + COB at PD1, in arm B. PFS from the start of run-in until PD1 was the primary endpoint (PFS1); secondary efficacy endpoints were OS, overall PFS (PFS2) and PFS3 (time from PD1 to PD after crossover, i.e. PD2) and best overall response rates (BORRs).
Results: The final analysis (median follow-up 57.0 months, interquartile range 22.7-63.0 months) confirmed longer PFS1 for continuous TT [arm A (69 patients) versus arm B (early switch, 66 patients); hazard ratio (HR) 0.61, 95% confidence interval (CI) 0.41-0.91, P = 0.006], but early switch to ICIs resulted in better long-term OS [4- and 5-year landmark OS 42% (95% CI 29% to 55%) and 40% (95% CI 27% to 53%) for arm A, and 53% (95% CI 38% to 65%) and 45% (95% CI 31% to 58%) for arm B; descriptive HR 1.17, 95% CI 0.71-1.91]. Absolute BORRs were 81% and 89%, respectively, with 15 (22%) and 19 (29%) patients achieving a complete response at least once along each sequence. At crossover, TT retreatment (arm B) resulted in higher PFS3 than second-line ICI (arm A).
Conclusions: Early switch to ICIs after TT run-in (arm B) led to an improved, although not statistically significant, 4- and 5-year landmark OS compared with arm A. No subgroups were identified for which a TT run-in provided clinical benefit. The number of patients developing brain metastasis and the time to brain metastasis were not improved by an early TT to ICI switch.
Keywords: crossover design; immunotherapy; melanoma; sequential therapy; targeted therapy.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Long G.V., Swetter S.M., Menzies A.M., Gershenwald J.E., Scolyer R.A. Cutaneous melanoma. Lancet. 2023;402:485–502. - PubMed
-
- Ny L., Hernberg M., Nyakas M., et al. BRAF mutational status as a prognostic marker for survival in malignant melanoma: a systematic review and meta-analysis. Acta Oncol. 2020;59:833–844. - PubMed
-
- Dummer R., Hauschild A., Lindenblatt N., et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v126–v132. - PubMed
-
- Michielin O., van Akkooi A.C.J., Ascierto P.A., Dummer R., Keilholz U., ESMO Guidelines Committee Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1884–1901. - PubMed
-
- Keilholz U., Ascierto P.A., Dummer R., et al. ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020;31:1435–1448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

