At-home Breast Oncology care Delivered with EHealth solutions (ABODE) study protocol: a randomised controlled trial
- PMID: 40345693
- PMCID: PMC12067776
- DOI: 10.1136/bmjopen-2024-091579
At-home Breast Oncology care Delivered with EHealth solutions (ABODE) study protocol: a randomised controlled trial
Abstract
Introduction: The COVID-19 pandemic disrupted healthcare delivery for patients with breast cancer. eHealth solutions enable remote care and may improve patient activation, which is defined as having the knowledge, skills and confidence to manage one's health. Thus, we developed the Breast Cancer Treatment Application (app) for patients and practitioners to use throughout the cancer care continuum. The app facilitates virtual assistance, delivers educational resources, collects patient-reported outcome measures and provides individualised support via volunteer e-coaches. Among newly diagnosed patients with breast cancer, we will compare changes in patient activation, other patient-reported outcomes and health service outcomes over 1 year between those using the app and Fitbit, and those receiving standard care and Fitbit only.
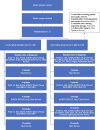
Methods and analysis: This randomised controlled trial will include 200 patients with breast cancer seen at a tertiary care cancer centre in Ontario, Canada. The intervention group (n=100) will use the app in addition to standard care and Fitbit for 13 months following diagnosis. The control group (n=100) will receive standard care and Fitbit only. Patients will complete questionnaires at enrolment, 6 and 12 months post-diagnosis to measure patient activation (Patient Activation Measure-13 score), distress, anxiety, quality of life and experiences with their care and information received. All patients will also receive Fitbits to measure activity and heart rate. We will also measure wait times and number of visits to ambulatory care services to understand the impact of the app on the use of in-person services.
Ethics and dissemination: Ethics approval was obtained on 6 January 2023. Protocol version 2.0 was approved on 6 January 2023. The trial is registered with ClinicalTrials.gov. Study findings will be disseminated via publication in a peer-reviewed journal and shared with participants, patient programmes and cancer awareness groups. The app has also been approved as a secure communication method at our trial institution, thus we are well-positioned to support future integration of the app into standard care through collaboration with our hospital network.
Trial registration number: NCT05989477.
Keywords: Breast surgery; Breast tumours; Randomized Controlled Trial; eHealth.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Virtual Care Task Force Report on virtual care in Canada: progress and potential. 2022. https://www.cma.ca/sites/default/files/2022-02/Virtual-Care-in-Canada-Pr... Available.
-
- World Health Organization . WHO guideline recommendations on digital interventions for health system strengthening. Geneva: World Health Organization; 2019. Executive summary.https://www.ncbi.nlm.nih.gov/books/NBK541888/ Available. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
