Effects of early factor XIII replacement in postpartum hae morrhage: study protocol for a multicentre, open-label, randomised, controlled, investigator-initiated trial
- PMID: 40345697
- PMCID: PMC12067823
- DOI: 10.1136/bmjopen-2025-100262
Effects of early factor XIII replacement in postpartum hae morrhage: study protocol for a multicentre, open-label, randomised, controlled, investigator-initiated trial
Abstract
Introduction: The primary objective of this trial is to evaluate the effect of replenishing coagulation factor XIII (FXIII) in women with postpartum haemorrhage (PPH) on measured blood loss (MBL). Based on earlier research, we hypothesise that the administration of FXIII leads to a significant reduction in postpartum blood loss.
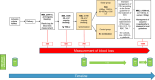
Methods and analysis: This is a randomised, controlled trial that will allocate eligible patients in the event of a PPH and after receiving tranexamic acid either to the treatment group, receiving FXIII, or to the control group (standard of care). The primary endpoint is the MBL within 24 hours using a standardised method. For the primary analysis, estimation of the OR under a proportional odds assumption is conducted simultaneously for all possible cut-off points. A corresponding estimate, along with a two-sided 95% CI and two-sided p value against the null hypothesis OR=1, is obtained from the Continuous Outcome Logistic Regression model. More than 7000 patients will be screened in order to include a total of 988 eligible patients into the trial. Secondary outcomes include the rate of adverse maternal outcomes related to PPH, the rate of women breastfeeding after PPH and the safety of the administration of FXIII in women with PPH. Dynamics of blood coagulation factors in women with PPH and their association with MBL will be assessed in specific centres. A preliminary overview on costs and potential savings through early treatment of PPH with FXIII is included in the analysis as well as a patient and public involvement report, asking for patients' personal experience during PPH in the main study centre.
Ethics and dissemination: Ethics approval was granted by the central ethics committee (Kantonale Ethikkommission Zürich/Switzerland) on 16 June 2024, reference number: BASEC 2024-00374. Results will be disseminated via open-access publication in a relevant medical journal.
Trial registration number: ClinicalTrials.gov ID NCT06481995.
Keywords: Bleeding disorders & coagulopathies; Postpartum Period; Pregnant Women; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: WK declared research support by CSL Behring to the institution (ZLM). CH received an unrestricted research grant from CSL Behring for an earlier investigator-initiated study on coagulation factors in 2015. All other authors have no completing interest to declare.
Figures


References
-
- WHO Guidelines Approved by the Guidelines Review Committee; 2012. WHO recommendations for the prevention and treatment of postpartum haemorrhage. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
