Therapeutic hyperthermia promotes lipid export and HSP70/90 during machine perfusion of human livers
- PMID: 40346031
- PMCID: PMC12064339
- DOI: 10.14814/phy2.70348
Therapeutic hyperthermia promotes lipid export and HSP70/90 during machine perfusion of human livers
Abstract
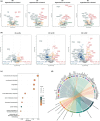
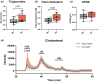
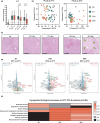
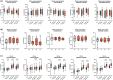
Liver transplantation is the only curative option for end-stage liver disease. Donor shortages necessitate the use of higher risk donor livers, including fatty livers, which are more susceptible to ischemia-reperfusion injury. Machine perfusion has improved graft utilization and is typically performed at hypothermic (8-12°C) or normothermic (35-37°C) temperatures. Here we studied the impact of mild hyperthermia (40°C) as a therapeutic intervention for fatty livers using in-depth proteomic and lipoprotein profiling of whole organ perfusion and precision-cut liver slices. We observed proteomic changes with metabolic alterations over time, evidenced by a significant increase in lipid export in whole organ perfusions. Furthermore, PCLS showed significant upregulation of metabolic processes and heat shock protein response after 24 h of hyperthermia. Machine perfusion under hyperthermic conditions may be a potential strategy to improve the utilization of fatty liver grafts, ultimately expanding the donor pool and improving transplant outcomes.
Keywords: hyperthermia; lipids; liver; machine perfusion; proteomics.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abbas, S. H. , Ceresa, C. D. L. , Hodson, L. , Nasralla, D. , Watson, C. J. E. , Mergental, H. , Coussios, C. , Kaloyirou, F. , Brusby, K. , Mora, A. , Thomas, H. , Kounali, D. , Keen, K. , Pollok, J. M. , Gaurav, R. , Iype, S. , Jassem, W. , Perera, M. T. P. R. , Hakeem, A. R. , … Friend, P. J. (2024). Defatting of donor transplant livers during normothermic perfusion—A randomised clinical trial: Study protocol for the DeFat study. Trials, 25(1), 386. - PMC - PubMed
-
- Archer, A. E. , Rogers, R. S. , von Schulze, A. T. , Wheatley, J. L. , Morris, E. M. , McCoin, C. S. , Thyfault, J. P. , & Geiger, P. C. (2018). Heat shock protein 72 regulates hepatic lipid accumulation. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 315(4), R696–R707. - PMC - PubMed
-
- Asrani, S. K. , Devarbhavi, H. , Eaton, J. , & Kamath, P. S. (2019). Burden of liver diseases in the world. Journal of Hepatology, 70(1), 151–171. - PubMed
-
- Aufricht, C. (2005). Heat‐shock protein 70: Molecular supertool? Pediatric Nephrology, 20(6), 707–713. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

